Detecting Preterm Labor with Raman Spectroscopy
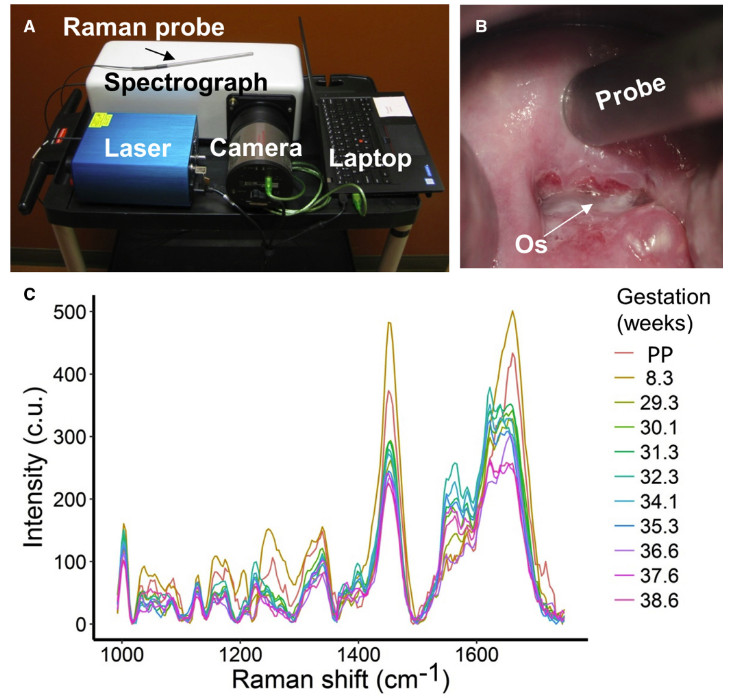 Prematurity is the second leading cause of neonatal mortality, leading to a myriad of complications, including delayed development and cerebral palsy. Currently, there is no way to accurately predict when a woman will deliver prematurely, making the prevention and treatment of preterm birth virtually impossible. While there are some populations at risk for preterm labor (patients with a history of previous preterm birth or uterine/cervical abnormalities), over half of all preterm births do not fall into any high-risk category.
Prematurity is the second leading cause of neonatal mortality, leading to a myriad of complications, including delayed development and cerebral palsy. Currently, there is no way to accurately predict when a woman will deliver prematurely, making the prevention and treatment of preterm birth virtually impossible. While there are some populations at risk for preterm labor (patients with a history of previous preterm birth or uterine/cervical abnormalities), over half of all preterm births do not fall into any high-risk category.
Researchers
Faculty
Post Doctoral Scholars and Graduate Students
-
-
Jennifer Bateman
- Rekha Gautam
Collaborators:
-
- Jeffery Resse, Vanderbilt University Medical Center
- J. Newton, Vanderbilt University Medical Center
- Kelly Bennett, Vanderbilt University Medical Center
- Jennifer Thompson, Vanderbilt University Medical Center
- Emad Elsamadicy, Vanderbilt University Medical Center
- Mack Goldberg, Vanderbilt University Medical Center
Publications
Herington JL, O'Brien C, Robuck MF, Lei W, Brown N, Slaughter JC, Paria BC, Mahadevan-Jansen A, Reese J. Prostaglandin-Endoperoxide Synthase 1 Mediates the Timing of Parturition in Mice Despite Unhindered Uterine Contractility. Endocrinology. 2018;159(1):490-505. PMID: 29029054.
McCarthy R, Martin-Fairey C, Sojka DK, Herzog ED, Jungheim ES, Stout MJ, Fay JC, Mahendroo M, Reese J, Herington JL, Plosa EJ, Shelton EL, England SK. Mouse models of preterm birth: suggested assessment and reporting guidelines. Biol Reprod. 2018. PMID: 29733339.
O'Brien CM, Vargis E, Rudin A, Slaughter JC, Thomas G, Newton JM, Reese J, Bennett KA, Mahadevan-Jansen A. In vivo Raman spectroscopy for biochemical monitoring of the human cervix throughout pregnancy. Am J Obstet Gynecol. 2018;218(5):528 e1- e18. PMID: 29410109.
Robuck MF, O'Brien CM, Knapp KM, Shay SD, West JD, Newton JM, Slaughter JC, Paria BC, Reese J, Herington JL. Monitoring uterine contractility in mice using a transcervical intrauterine pressure catheter. Reproduction. 2018;155(5):447-56. PMID: 29500186.
O'Brien CM, Herington JL, Brown N, Pence IJ, Paria BC, Slaughter JC, Reese J, Mahadevan-Jansen A. In vivo Raman spectral analysis of impaired cervical remodeling in a mouse model of delayed parturition. Sci Rep. 2017;7(1):6835. PMID: 28754971.
Robuck M, O'Brien C, Reese J, Herington J. Measuring In Vivo Uterine Contractile Activity of Pregnant Mice via a Transcervical Intrauterine Pressure Catheter (IUPC). Reprod Sci. 2017; Volume: 25 issue: 1_suppl, page(s): 55A-324A. https://doi.org/10.1177/1933719118759999
O'Brien CM, Brown N, Rudin A, Slaughter C, Bennett KA, Reese J, Mahadevan-Jansen A. Detection of Biochemical Changes in the Cervix During Induction of Labor in Human Subjects and Mouse Models Using In Vivo Raman Spectroscopy. Reprod Sci. 2016; Volume: 23 issue: 1_suppl, page(s): 51A-344A https://doi.org/10.1177/1933719116641257
Pence I, Mahadevan-Jansen A. Clinical instrumentation and applications of Raman spectroscopy. Chem Soc Rev. 2016;45(7):1958-79. PMID: 26999370.
O'Brien CM, Vargis E, Slaughter C, Rudin AP, Herington JL, Bennett KA, Reese J, Mahadevan-Jansen A. Characterization of human cervical remodeling throughout pregnancy using in vivo Raman spectroscopy. Proc Spie. 2015; doi: Artn 93032f10.1117/12.2077775. https://www.spiedigitallibrary.org/conference-proceedings-of-spie/9303/93032F/Characterization-of-human-cervical-remodeling-throughout-pregnancy-using-in-vivo/10.1117/12.2077775.short?SSO=1
O'Brien CM, Vargis E, Paria BC, Bennett KA, Mahadevan-Jansen A, Reese J. Raman spectroscopy provides a noninvasive approach for determining biochemical composition of the pregnant cervix in vivo. Acta Paediatrica. 2014;103(7):715-21. PMID: 24628401.
Vargis E, Byrd TT, O'Brien CM, Logan Q, Khabele D, Mahadevan-Jansen A. In Vivo Raman Spectroscopy Detects Cervical Intraepithelial Neoplasia with High Accuracy in Diverse Population. Reprod Sci. 2014 Volume: 21 issue: 3_suppl, page(s): 71A-418A. https://doi.org/10.1177/1933719114528275
O'Brien C, Vargis E, Borwn N, Reese J, Paria BC, Mahadevan-Jansen A. In Vivo Detection of Biochemical Change in the Pregnant Cervix in Humans and Mouse Models. Reprod Sci. 2013; Volume: 20 issue: 3_suppl, page(s): 61A-341A. https://doi.org/10.1177/1933719113482088
