Heme Biosynthesis is controlled by reversible feedback mechanism inside the mitochondrial matrix
By Shelby A. Harris
 Heme is vital for life. It is needed for oxygen transport, drug detoxification, and many other biological functions. Regulation is key, too much or too little heme can cause a host of problems in the body. Aminolevulinic acid synthase (ALAS) is heme’s rate-limiting enzyme that functions by the condensation of glycine and succinyl-CoA to produce aminolevulinic acid. Two isoforms are found in humans, denoted as ALAS1 and ALAS2, the latter of which controls 85 to 90% of heme synthesis. Most disease-causing mutations are found in this enzyme and recent studies suggest that ALAS2 is more widely expressed than previously thought.
Heme is vital for life. It is needed for oxygen transport, drug detoxification, and many other biological functions. Regulation is key, too much or too little heme can cause a host of problems in the body. Aminolevulinic acid synthase (ALAS) is heme’s rate-limiting enzyme that functions by the condensation of glycine and succinyl-CoA to produce aminolevulinic acid. Two isoforms are found in humans, denoted as ALAS1 and ALAS2, the latter of which controls 85 to 90% of heme synthesis. Most disease-causing mutations are found in this enzyme and recent studies suggest that ALAS2 is more widely expressed than previously thought.
Dr. Iva Chitrakar, a postdoc in the lab of Dr. Breann Brown, states that this underscores the critical need to understand ALAS2’s regulation. Little is known about ALAS within the mitochondrial matrix, where heme biosynthesis occurs. Dr. Chitrakar identified a reversible mechanism by which heme inhibits its own synthesis by affecting mature mitochondrial ALAS2 activity. Inactivating ALAS2 is inactivated in the presence of heme stress in order to reduce heme synthesis, a new form of negative feedback in heme biosynthesis.
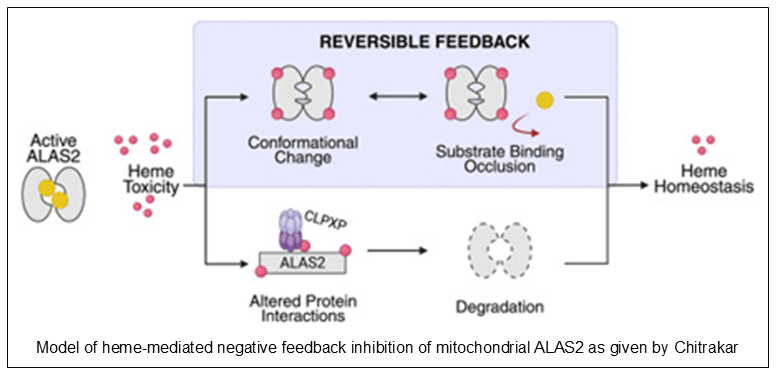 The regulation of ALAS2 is an intricate, multifaceted process where heme acts as an allosteric effector to maintain cellular homeostasis. These findings support a model where the presence of multiple heme-binding sites within the enzyme likely serves as a fail-safe mechanism so that ALAS2 can still interact with heme even if one site fails. Since the ALAS2 homodimer contains multiple nonequivalent heme-binding sites, the enzyme can redundantly tune its activity, likely by inducing conformational changes that block substrate binding or by recruiting the CLPXP protease for targeted degradation. This inhibitory mechanism may also extend to the “heme synthesis metabolon,” a complex of mitochondrial proteins that optimizes metabolic flux. By combining this rapid allosteric inhibition with slower, irreversible degradation, the cell can precisely calibrate heme production to support its vital biological functions.
The regulation of ALAS2 is an intricate, multifaceted process where heme acts as an allosteric effector to maintain cellular homeostasis. These findings support a model where the presence of multiple heme-binding sites within the enzyme likely serves as a fail-safe mechanism so that ALAS2 can still interact with heme even if one site fails. Since the ALAS2 homodimer contains multiple nonequivalent heme-binding sites, the enzyme can redundantly tune its activity, likely by inducing conformational changes that block substrate binding or by recruiting the CLPXP protease for targeted degradation. This inhibitory mechanism may also extend to the “heme synthesis metabolon,” a complex of mitochondrial proteins that optimizes metabolic flux. By combining this rapid allosteric inhibition with slower, irreversible degradation, the cell can precisely calibrate heme production to support its vital biological functions.
You can find more about this study in the Journal of Biological Chemistry!
Leave a Response
You must be logged in to post a comment