CSB Research: Crystallizing P450 complexes leads to evolutionary implications

Did you know that the cytochrome P450 enzyme family is one of the largest found in nature? These enzymes are found in all domains of life and catalyze electron transport for diverse processes like sterol biosynthesis and the activation/detoxification of drugs. Drs. Galina Lepesheva and Jarrod Smith recently published a structural characterization of the CYP51-ferredoxin fusion protein found in Methylococcus capsulatus, thought to be an evolutionary ancestor of all P450s.
Using x-ray crystallography, the ROSETTA suite and molecular dynamics simulations, they showed that the M. capsulatus fusion protein bound substrate more tightly but only formed an ensemble of transient complexes between P450 and ferredoxin in comparison to eukaryotic CYP51 enzymes.
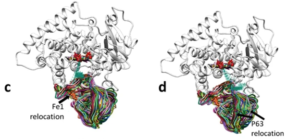
The authors suggest that this leads to a decreased catalysis rate, which could be a trade-off for guaranteed complex formation between the P450 and ferredoxin in the protein-rich environment of M. capsulatus. Furthermore, these data suggest an evolutionary path for P450s that increases catalysis rate by increasing binding specificity on P450s for the redox partner.
Read more about it here! ~ Steven D. Walker
Leave a Response
You must be logged in to post a comment