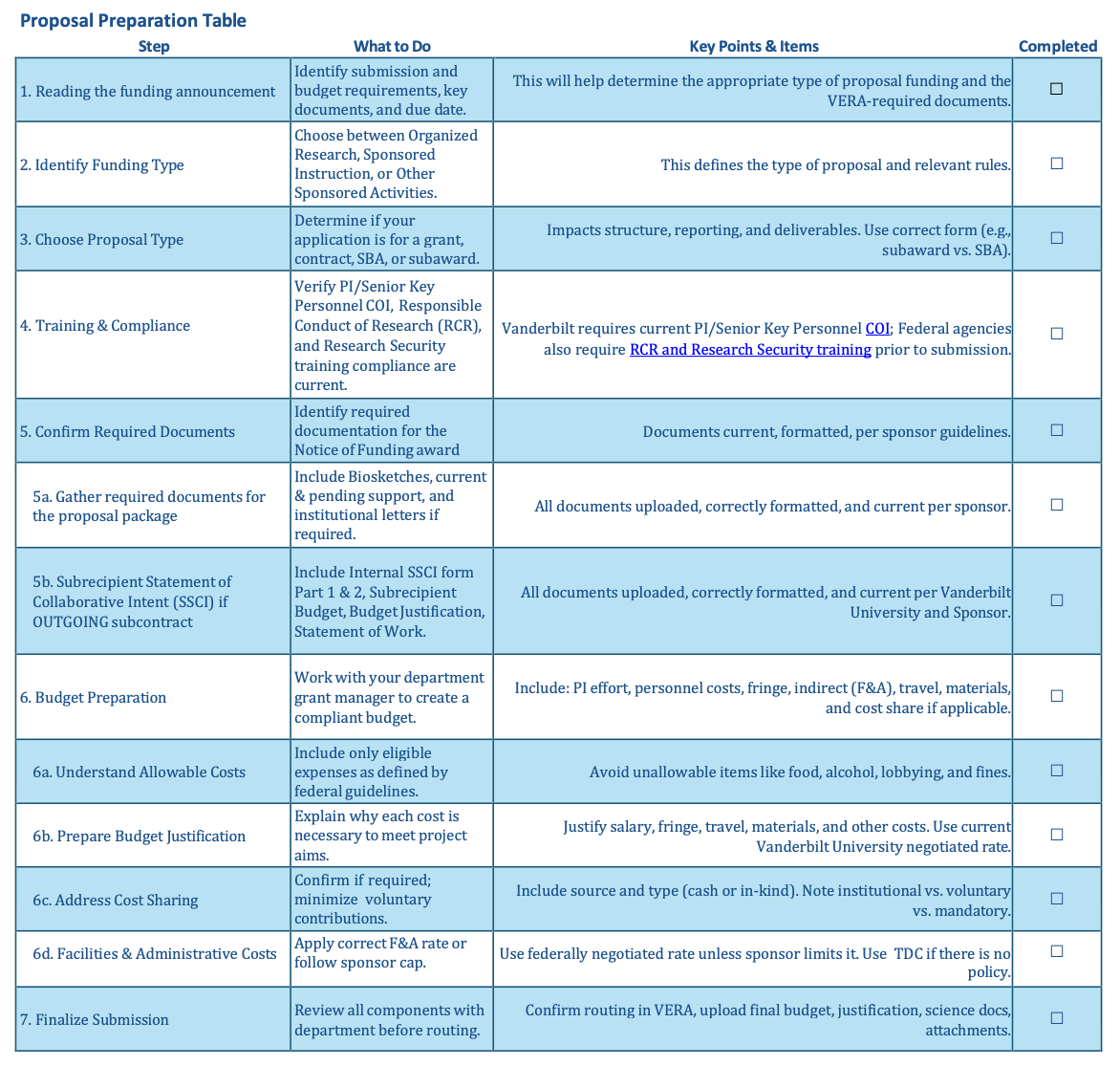
Preparing a Proposal
Vanderbilt researchers can access numerous funding options from various sources, including the U.S. federal government, foundations, foreign governments, non-profit organizations, and corporations. The Sponsored Programs Administration (SPA) provides valuable knowledge and services to assist departments throughout the proposal process. Together - they help transform initial ideas into well-crafted proposals, maximizing the university's chances of securing research funding.
-
SPA Fact Sheet & Getting Started
SPA Fact Sheet
Most proposals require some general information about Vanderbilt to be included in the submission. This sheet includes a variety of information including the institutional address, official institutional contacts, and organization-specific information that may be requested by sponsors.
Roles & Responsibilities Matrix
Getting Started
The Principal Investigator (PI) normally finds a funding opportunity to apply to and will then reach out to their unit’s grant management team to get started on the proposal submission. It is important to review the solicitation/funding opportunity announcement and any applicable sponsor guidelines for the submission, establish timelines and communication preferences with the research team, and confirm the process to submit early in the proposal preparation process. Links to SPA resources related to getting started on a funding proposal are included below.
Requesting access to common external sponsor portals
Provides basic instructions for gaining access to standard external portals for grant management and proposal submission.VERA New Funding Proposal Checklist
VERA BASICS TRAINING VIDEOS
New Sponsors & New Organizations: the process (FAQ) - (only use the Request a New Sponsor online form to add sponsors after you’ve confirmed they are not already in the system)
The FAQ explains the process for requesting a new organization is added to both VERA and Oracle, for use in VERA for Proposals, Awards and Agreements. ONLINE FORM: Request a new Sponsor in VERAPROPOSAL DEVELOPMENT AND SUPPORT SERVICES
Separate from SPA, Research Development and Support (RDS) provides pre-award services that include identifying funding opportunities, proposal coordination, and grant writing assistance. Contact RDS via their intake form or email them directly at rds@vanderbilt.edu to request their services.
GRANT REQUIREMENTS HIERARCHY AT VANDERBILT UNIVERSITY
At Vanderbilt University, all grant proposals must first and foremost comply with university-wide and school-specific policies. While it is essential to follow the Sponsor’s Notice of Funding Opportunity (NOFO) / solicitation guidelines, proposals must be developed in alignment with institutional policies. In cases of conflict between guidelines, follow the below hierarchy. For example, if the NOFO / solicitation requirements and the general sponsor requirements conflict, follow the solicitation guidance:

-
PI Eligibility
-
Preparing a Proposal
-
Research Types
Below are the three research types supported at Vanderbilt University. The research type may determine the correct indirect cost rate to apply and impacts reporting, so it is important to select the correct research type in VERA when building your proposal.
-
Funding Mechanisms
Grants
Description: An arrangement under which there is a transfer of funds, property, services or anything of value from the sponsor to the institution to assist the institution in reaching a particular goal or public purpose.
- PI defines the project - usually fairly loosely - Scope of Work or Proposal is cited in award
- Sponsor retains the right to revoke the award and unused funds revert back to sponsor
- Has a defined period of performance
- Reports are normally on an annual basis
- Supports further knowledge in a particular subject area or field of research
- VU owns IP
- Publications are not restricted
- "Best efforts" are used in completing research
- Benefit is normally to the grantee/PI by furthering their own purposes or programs
- IRS includes scholarships, fellowships, internships, prizes and awards
- May qualify as charitable contribution depending on source of funds
Contracts
Description: A mechanism for the procurement of a specific service or product with specific obligations for both the buyer and the seller. Creates a quid pro quo relationship.
- Sponsor or Sponsor and PI jointly define Scope of Work
- Sponsor retains the right to terminate the contract
- Reports are often done more frequently than annually
- Publication may require review/approval of the sponsor
- Benefit is normally to the sponsor - anticipates an economic benefit as a result of the activity to be conducted
- Contractor generally is required to produce a work product or deliverable (it's possible this is only a report of findings)
- Contractors are paid only if the deliverable is accomplished
- Most involve some supervision or control by sponsor (on expenditures and/or deliverable)
- Provides expertise/knowledge to solve a problem
Sponsored Billing Agreement (SBA)
Description: A Billing Agreement is a short and efficient mechanism through which VU and VUMC can contract for limited work to be performed and to effectuate payments. There are a few things to know:
- Sponsored Billing agreements are only for inter-institutional relationships between VU and VUMC.
- A Sponsored Billing Agreement cannot substitute for a subcontract. If a faculty member is performing substantive scientific work on a project, a subaward, not a Sponsored Billing Agreement, is the appropriate mechanism.
- Funding for Sponsored Billing Agreements only comes from a grant or a contract (sponsored awards).
- Sponsored Billing agreements provide for reimbursement of direct costs. They do not have indirect costs.
Subawards
Description: A formal written agreement made between VU and another institution or organization to perform a significant portion of a project.
- INCOMING Subaward: Issued under a prime award (grant, contract or cooperative agreement) where a portion of the scope of work is delegated by the prime award recipient to Vanderbilt University
- OUTGOING Subaward: Issued under a prime award (grant, contract or cooperative agreement) where a portion of the scope of work is delegated by Vanderbilt University to a subrecipient
-
Common Proposal Elements
Standard Documents:
Project Summary / Abstract: This is a brief overview of the project. Depending on the sponsor's requirements, specific information or sections may need to be included.
Project Description / Narrative: This is the most important document or section as it is the description of the project. The project description always has page or character limitations, which are described in the sponsor’s guidelines or specific funding opportunity announcement (FOA).
Budget: The budget is the financial interpretation of the proposed project. It should contain all costs that are related to the proposed work. Sponsors often have limitations on the amount and type of items that can be requested in a budget, and the budget also must conform to Vanderbilt and individual School’s policies.
NIH modular budget and detailed budget examples
Budget Justification: This is a document or proposal section that includes a description of each budgeted item, how the project will benefit from the item, and how the amount requested was derived. It should also include any information required by the sponsor.
Biographical Sketch / CV: Most proposals require either a Biographical Sketch or a CV for all senior personnel on the project. Depending on the sponsor, a specific format and/or page limitation may be required for this document. This document is one way to show reviewers that the PI and their project team are qualified to complete the proposed work.
Documents that may also be required:
Facilities: This document contains a description of the research environment and any Vanderbilt-provided resources the project will be using for the project (things like Libraries, Equipment, Office Space).
Data Management Plan: This is a description of how the project team will collect, store, and disseminate project data.
Letters of Support or Commitment: These letters may be required within the FOA or are needed to show that groups the project will be working with outside Vanderbilt agree to serve on the project.
Additional Documents (depending on the sponsor or FOA requirements): Beyond these items, there may be additional required documents or information depending on the sponsor or the FOA.
-
Proposal Budget Guidance
The proposal budget is a financial representation of the work to be undertaken. It provides a detailed breakdown of the costs necessary to achieve the proposed outcomes. A well-developed budget should align with and reflect the scope of work outlined in the proposal.
Grants Managers in Schools and Departments work with the Principal Investigator (PI) to develop an accurate, complete budget that adheres to the sponsor’s and University’s guidance and policies.
Cost Principles
Allowable Costs
To be charged to a sponsored project, costs must comply with federal, sponsor, and institutional guidelines, meeting six key criteria: they must be reasonable (reflecting prudent judgment), allocable (benefiting the project specifically or in measurable proportion), consistently treated (handled uniformly across funding sources), necessary (essential to the project), compliant with award-specific and federal regulations, and incurred within the project's designated period.
Unallowable Costs
Certain costs are deemed unallowable by the federal government and cannot be charged to sponsored projects. These include expenses related to advertising and public relations (except for personnel recruitment), alcoholic beverages, alumni activities, bad debt, donations, entertainment, fines and penalties, fundraising, personal use items, interest expenses, lobbying, civic or community organization memberships, and proposal preparation costs such as time, printing, and postage.
Guidelines for Budgeting and Charging Direct Costs on Sponsored Projects
Uniform Guidance for Cost Principles
Salary Cap Information
Since 1990, Congress has imposed salary caps on grants, contracts, and cooperative agreements from agencies such as the NIH, AHRQ, and SAMHSA, with the NSF also limiting compensation for principal investigators and senior personnel. These salary caps apply to all applicable awards received by Vanderbilt University, including those involving subcontracts or awarded to subcontractors. For further guidance, individuals are advised to contact their Sponsored Programs Administrator.
Salary Cap Procedures & Guidance
NIH Salary Cap Summary - NIH Salary Cap Levels by Year
Facilities & Administrative (F&A) costs
F&A costs, previously known as indirect costs, are general organizational expenses that support research and services but are not tied to specific projects. Vanderbilt University applies a federally negotiated F&A rate to Modified Total Direct Costs (MTDC), which include most direct costs such as salaries, fringe benefits, materials, and the first $25,000 of subawards or subcontracts. MTDC excludes certain items like equipment over $5,000, federal financial aid, patient care, tuition remission, capital expenditures, and participant support costs. This MTDC base is used for all proposals unless a sponsor’s guidelines specify otherwise, in which case documentation must be attached when using a reduced F&A rate.
Facilities and Administrative (F&A) Cost Rates
Cost-Sharing
Cost sharing, or matching funds, refers to covering a portion of a sponsored project's costs using non-sponsor resources, and it should be minimized when possible. There are three types: mandatory cost sharing, required by the sponsor as a condition of the award; voluntary committed cost sharing, which is not required but included in the proposal and must be honored if funded; and voluntary uncommitted cost sharing, which is not required or included in the proposal budget but may still benefit the project. Cost-sharing funds can come from university resources or external partners, either as direct cash contributions or in-kind donations such as services or supplies, provided their value can be reasonably determined.
Cost Sharing on Sponsored Awards Policy (search for cost-sharing)
Cost Sharing on Sponsored Awards Procedures
Standard Budget Categories
These are common budget categories seen across various funding opportunities: Salaries & Wages (Personnel), Fringe Benefits, Equipment, Travel, Participant/Trainee Support Costs (PSC), Materials & Supplies, Subawards/Consortium/Contractural Costs, Other Direct Costs, and Facilities & Administrative Costs (F&A)
-
Budgeting Tools and Resources
Vanderbilt Research Finance Budget Resources
Guidelines for Budgeting and Charging Direct Costs on Sponsored Projects
Salary Cap Procedures & Guidance
Facilities and Administrative (F&A) Cost Rates
Cost Sharing on Sponsored Awards Policy (Search Vanderbilt University's Policy Portal)
Cost Sharing on Sponsored Awards Procedures
Budgeting of Tuition for Graduate Student Research Assistants at Vanderbilt
SPA/VERA Budget Preparation Resources
Guide to Proposal Budget Preparation
Principles of Budgeting: Sponsored Project Personnel
VERA-based Budget Template Documents
VERA Instructions for Exceptional Budgets
Federal and Sponsor-Specific Budget Resources
-
Proposal Review
SPA Proposal Review
SPA completes their review of the proposal once it has successfully routed and reviewed in VERA. Best practice is to not route the VERA record until all administrative portions of the proposal are final and available to the SPA Specialist for review. The items considered administrative are included in the below Proposal Review Requirements guide.
For a proposal to receive a full review by your SPA Specialist, it must be received with all administrative items finalized at least 3 full business days in advance of the submission deadline. Additional information on the level of proposal review based on the date they are received by SPA is found in the below Proposal Review Requirements guide.
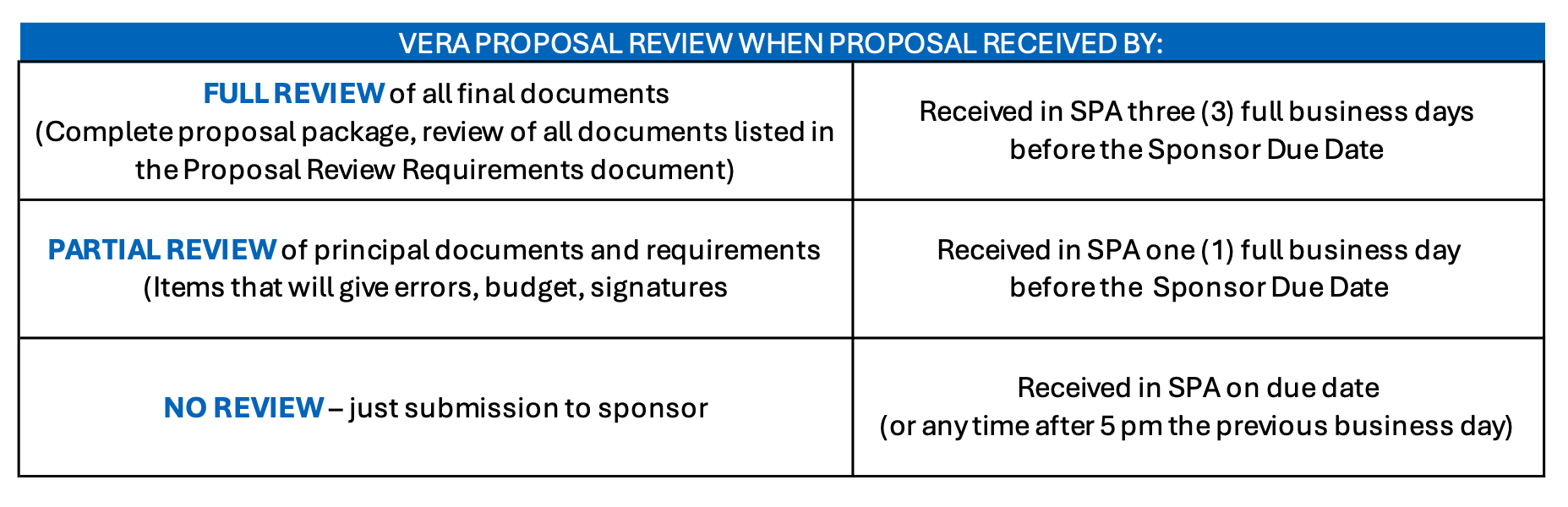
SPA Proposal Review Requirements
Proposal Submission
SPA is often the Vanderbilt unit submitting the proposal to the sponsor. If this is the case, the grants manager will work closely with their SPA Specialist to have the proposal submitted after the administrative review is completed and all final documents are included in the proposal submission. If SPA is not the submitter, the grants manager will follow the application instructions to submit the proposal to the sponsor and log a copy of the final submitted proposal in VERA. If a non-governmental portal is involved in the submission process, grants managers should check with their SPA Specialist to confirm if they will work with SPA, RDS, or independently to access the portal and submit.