Closing out an Award
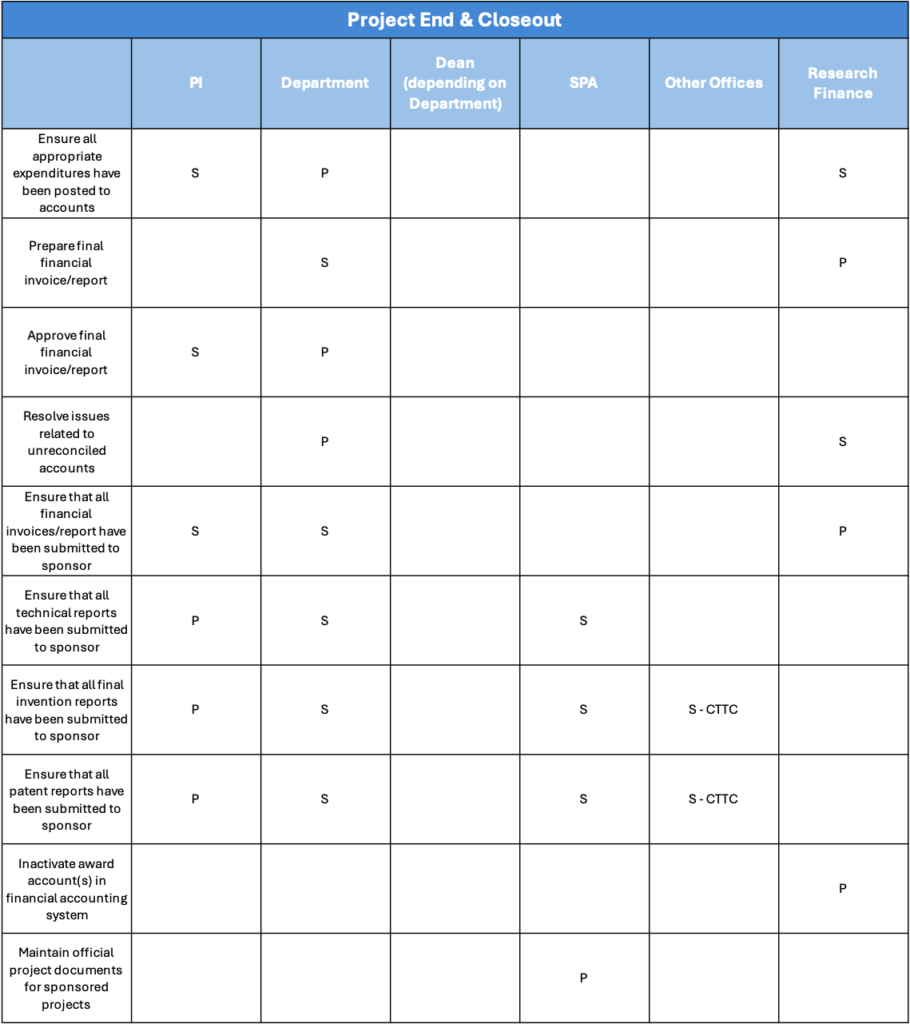
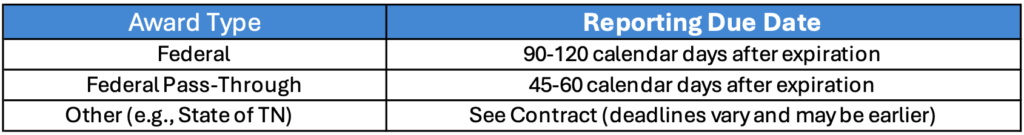
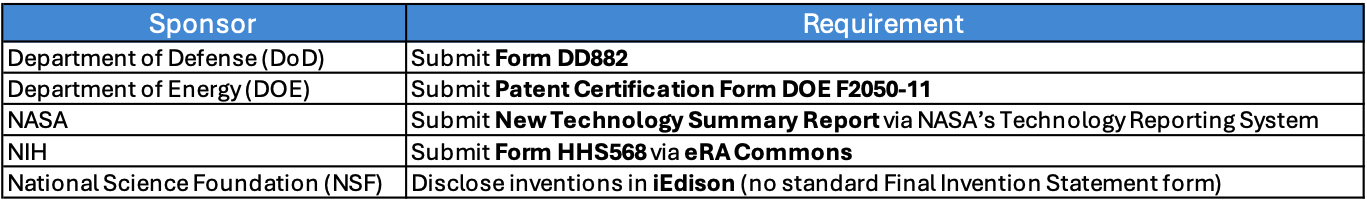
The closeout process is the final administrative step of a sponsored award, ensuring all financial, technical, and compliance obligations are met. It includes submitting final reports (financial, technical, and invention statement), reconciling expenditures, verifying deliverables, and confirming all sponsor and institutional requirements are satisfied before formally closing the award. The Principal Investigator (PI), Grants Manager, Sponsored Programs Administration (SPA) and Research Finance offices all have a part to play in closing out an award. Closeout requirements vary by sponsor, so please check with your Department, Research Finance, and SPA to ensure we follow the appropriate procedures.