A new toxin toolkit for C. diff

Clostridioides difficile (C. diff) is a leading cause of diarrhea and healthcare-associated infections in the United States and recognized as an urgent threat by the Centers for Disease Control. Virulence of C. diff infections is believed to rely primarily on the production of two toxins: TcdA and TcdB. TcdA and TcdB are large homologous proteins which inhibit Rho-family GTPases.
While many vaccine trials have focused on the use of toxoid antigens, little clinical success has been achieved and treatment options for C. diff infection are limited. The need to generate broadly neutralizing antibody responses has necessitated a clearer understanding of toxin sequences and structures, especially with the recent knowledge that TcdB sequences can vary between circulating clinical strains. Further, there are no methods available to quantify TcdA and TcdB concentrations in research studies.
In this study, Shannon Kordus, a postdoctoral research fellow, and Heather Kroh, a research assistant professor, both in the Lacy lab, work in collaboration with the Spiller and Wadzinski labs to investigate TcdA and TcdB toxins. Nanobodies against non-toxic point mutations in TcdA and TcdB were generated from alpacas and assessed for toxin neutralization in a cell viability assay. Structural analysis was then conducted with negative stain EM to determine the location of nanobody binding. TcdA and TcdB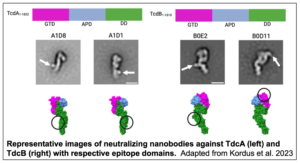 are both composed of four domains: the N-terminal glucosyltransferase domain (GTD), the autoprocessing domain (APD), the delivery domain (DD) and the combined repetitive oligopeptides (CROPs) domain.
are both composed of four domains: the N-terminal glucosyltransferase domain (GTD), the autoprocessing domain (APD), the delivery domain (DD) and the combined repetitive oligopeptides (CROPs) domain.
Previous research has focused primarily on the CROPs domain of TcdA due to its role in host cell receptor binding. However, the majority of identified TcdA neutralizing nanobodies were found to bind the delivery domain. In contrast, TcdB neutralizing nanobodies bound multiple different domains. Neutralizing antibodies for both toxins were subsequently used to develop a sandwich ELISA assay capable of quantitatively measuring TcdA and TcdB concentrations in vitro and in the cecal and fecal contents of infected mice. Together, these assays will provide an avenue for further research into C. diff infection virulence, symptomology and treatment.
Read more about these exciting new tools here! ~ Cameron I. Cohen
Leave a Response
You must be logged in to post a comment