Srivastava, Aruesha., Shaik, Neha., Lu, Yunrui., Chan, Matthew., Diallo, Alos B., Zavras, John P., Han, Serin., Punshon, Tracy., Jackson, Brian Phillip., Vahdat, Linda T., Liu, Xiaoying., Mittal, Vivek., Lau, Ken S., Gui, Jiang., Vaickus, Louis J., Hoopes, Jack., Kolling, Fred W., Perreard, Laurent., Marotti, Jonathan Douglas., & Levy, Joshua J. (2025). Integration of elemental imaging and spatial transcriptomic profiling for proof-of-concept metals-based pathway analysis of colon tumor microenvironment. Metallomics, 17(10), mfaf034. https://doi.org/10.1093/mtomcs/mfaf034
Metals such as iron and copper play important roles in cancer biology, but how they interact with genes, cell types, and tumor growth processes is still not well understood. In the past, metals and genes were usually studied separately, which made it hard to see how they influence each other. New technologies—like spatial transcriptomics for measuring gene activity in specific locations and elemental imaging for mapping metals across tissue—now make it possible to study these relationships simultaneously, though combining these data remains challenging.
In this proof-of-concept study, we examined metal-related signaling in the tumor microenvironment of a single colorectal cancer (CRC) tumor using a spatial multimodal workflow. This approach integrated elemental imaging, gene expression patterns, cell composition, and histopathology to identify metal-linked biological pathways. Our early results showed notable relationships—for example, high iron levels were found in areas with mesenchymal-like cells at the tumor’s growing edge, along with increased expression of genes involved in epithelial-to-mesenchymal transition and extracellular matrix remodeling. We also observed that regions with high copper concentrations overlapped with zones of active tumor growth and were associated with increased expression of immune response genes.
These findings, while based on a single sample, demonstrate that combining elemental imaging with spatial transcriptomics can successfully reveal how metals and gene activity interact within tumors. Applying this workflow to larger patient groups in the future may help uncover new biomarkers and therapeutic targets related to metal-driven tumor progression.
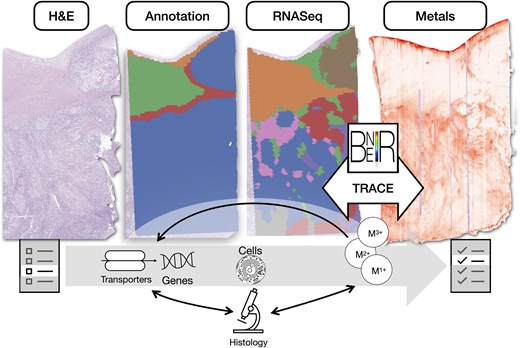
Figure 1.
Overview: spatial integration of spatial elemental imaging and spatial transcriptomics can reveal genes associated with metal bioaccumulation within specific tissue architectures, shedding light on metals-related pathways and cellular changes associated with tumorigenesis; BNEIR: biomedical national elemental imaging resource; TRACE: tissue region analysis through co-registration of elemental maps.