Insights into the regulation of Huntingtin-associated proteins
Huntington’s disease is a genetic neurodegenerative disease that causes wide-ranging issues with motor skills and cognitive abilities. The primary cause of the disease has been identified as mutations in the protein huntingtin (Htt). Htt interacts with over 200 other proteins and plays a role in cell signalling and trafficking.
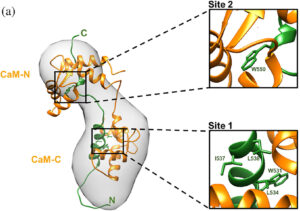
The Chazin group recently published an article in Protein Science that looks at a domain of the protein hPrp40A, an interacting partner of Htt. Both Htt and hPrp40 are modulated by the calcium sensor protein calmodulin (CaM), and this study explores the interaction of CaM with the FF3 domain of hPrp40 in the context of Prp40-Htt function.
A variety of biophysical techniques were used to show that CaM engages both domains while binding FF3, and the binding requires the partial unfolding of the FF3 domain to expose key Trp anchor residues. A consensus model was proposed where CaM binds to an extended non-globular state of FF3, coupled to its transient unfolding.
This study provides a piece of the puzzle that is the Htt-protein interaction network. It also opens possible therapy avenues for Huntington’s disease, which needs further exploration.
Read the complete article here! ~Swati Balakrishnan
Leave a Response
You must be logged in to post a comment