Quoc-Huy Trinh, Vincent; Ankenbauer, Katherine E.; Torbit, Sabrina M.; Taranto, Christopher P.; Liu, Jiayue; Batardiere, Maelle; Kumar, Bhoj; Carlo Maurer, Hans; Revetta, Frank L.; Chen, Zhengyi; Kruse, Angela R.S.; Judd, Audra M.; Copeland, Celina; Wong, Jahg; Ben-Levy, Olivia; Jarvis, Brenda; Brown, Monica E.; Brown, Jeffrey W.; Das, Koushik K.; Makino, Yuki; Spraggins, Jeffrey M.; Lau, Ken S.; Azadi, Parastoo; Maitra, Anirban; Tan, Marcus Chuan Beng; & DelGiorno, Kathleen E. (2025). Mutant GNAS drives a pyloric metaplasia with tumor suppressive glycans in intraductal papillary mucinous neoplasia. Cell Reports, 44(12), 116684. https://doi.org/10.1016/j.celrep.2025.116684
Intraductal papillary mucinous neoplasms (IPMNs) are cystic lesions of the pancreas and are established precursors to pancreatic ductal adenocarcinoma (PDAC), one of the most lethal solid cancers. Although about 90 percent of IPMNs are detected before PDAC develops, there are no reliable biomarkers to distinguish benign from malignant lesions, which leads to many unnecessary surgical resections. Recent work has shown that pancreatic precancer often adopts a pyloric phenotype, meaning the cells resemble those found in the pylorus of the stomach.
To identify the regulators of this cellular plasticity, the study analyzed cell lines, organoids, mouse models of IPMNs, and human patient samples using multiplex immunostaining, RNA sequencing, glycosylation profiling, and computational analyses. These approaches revealed that the GNASR201C mutation promotes an indolent, or slow growing, phenotype in IPMNs by enhancing a differentiated pyloric program through the transcription factors SPDEF and CREB3L1. This pyloric state is associated with specific patterns of glycosylation, or sugar modifications on proteins.
GNASR201C acts as a glycan rheostat, meaning it shifts the balance of cell surface sugars by increasing LacdiNAc structures while reducing pro tumorigenic acidic Lewis epitopes. This glycan switch suppresses cancer cell invasion and slows disease progression. Importantly, LacdiNAcs and 3′-sulfo-LeA/C glycans are mutually exclusive, and their presence may serve as biomarkers to stratify IPMN patients by cancer risk and guide surgical decision making.
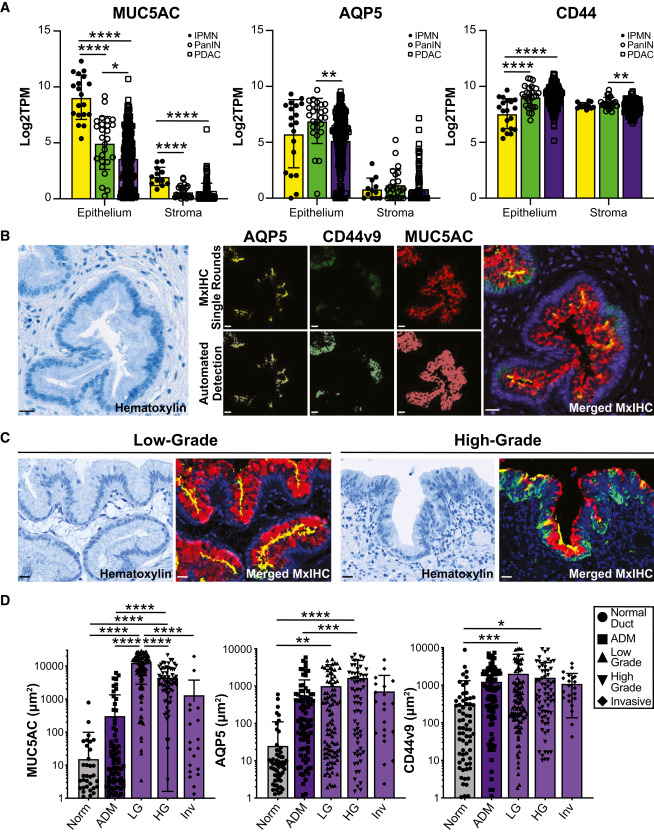
Figure 1 Human IPMN are characterized by pyloric metaplasia
(A) Bar plots comparing the expression of MUC5AC, AQP5, or CD44 in the epithelium and stroma in IPMNs (yellow, n = 19), PanIN (green, n = 26), and PDAC (purple, n = 197 epithelium, 124 stroma).19
(B) Hematoxylin staining and pseudo-colored immunohistochemical staining for MUC5AC (red), AQP5 (yellow), or CD44v9 (green), top row, and automated detection of signal (bottom row) by QuPath to merge MxIHC data.21Scale bars, 20 μm.
(C) Examples of hematoxylin staining or merged MxIHC of low-grade (left) or high-grade (right) IPMNs. Scale bars, 20 μm.
(D) Quantification of staining in (B)–(C) for 40 IPMN patients, including normal ducts (ND; n = 95–109), acinar-to-ductal metaplasia (ADM; n = 109), low-grade IPMN (LG; n = 110), high-grade IPMN (HG; n = 70), and invasive IPMN (Inv; n = 22).
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.005; and ∗∗∗∗p < 0.001.