Cronin, Alicia E., Combes, Anna J.E., Sweeney, Grace., Prock, Logan E., Houston, Delaney C., Stuart, Isabella., Stubblefield, Seth., McKnight, Colin David., Bagnato, Francesca R., O’Grady, Kristin P., & Smith, Seth A. (2025). Relaxation-compensated chemical exchange saturation transfer MRI in the cervical spinal cord at 3T: An application in multiple sclerosis. NeuroImage: Reports, 5(4), 100298. https://doi.org/10.1016/j.ynirp.2025.100298
Multiple sclerosis (MS) is an autoimmune disease that damages the central nervous system, particularly by destroying the protective myelin around nerves. Studying changes in the spinal cord could help us understand why MS causes neurological problems and clinical symptoms. However, conventional MRI does not detect subtle molecular changes in tissue. Chemical exchange saturation transfer (CEST) is an MRI technique that can measure biochemical changes in tissue with high sensitivity and without the need for contrast agents. In practice, CEST signals are influenced by other effects—such as semi-solid magnetization transfer (MT), direct water saturation, and T1 relaxation—that can be altered in MS, so these confounding factors must be removed to accurately quantify changes.
In this study, 53 people with relapsing-remitting MS (pwRRMS) and 45 healthy controls were scanned at 3 T to measure amide and nuclear Overhauser enhancement (NOE) CEST effects in the cervical spinal cord. Using a method called Lorentzian fitting, we removed confounding effects and calculated the apparent exchange-dependent relaxation (AREX) contrast. Comparing uncorrected and corrected AREX contrasts across tissue types and groups revealed that AREX NOE differed significantly in lesions compared to normal-appearing white matter in pwRRMS. People with MS also showed greater variability in both CEST contrasts than healthy controls. A subgroup analysis based on neurological disability showed that AREX amide differed significantly between pwRRMS patients with and without disability.
These findings highlight the importance of correcting for confounding effects in CEST imaging to isolate true biochemical changes in the cervical spinal cord. Doing so provides a more specific characterization of tissue pathology and helps link molecular changes to disease severity in MS.
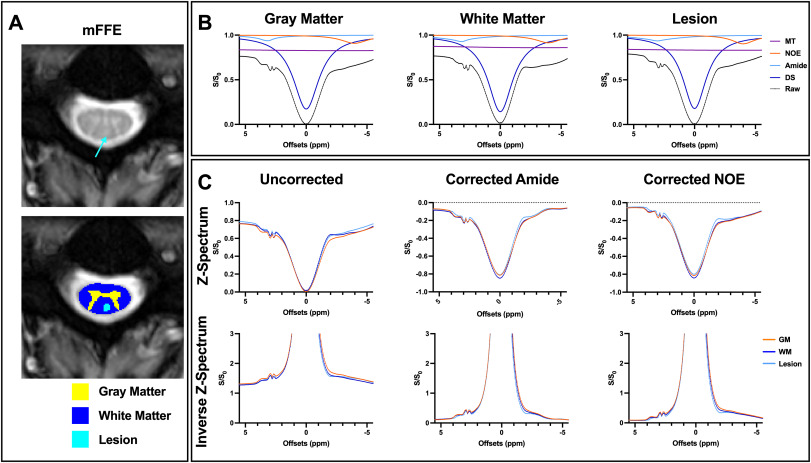
Fig. 1. Example data from one multiple sclerosis (MS) participant (32 years, female, 0 Expanded Disability Status Scale (EDSS) score, 1.5 years disease duration). A. Representative anatomical multi-echo, gradient echo (mFFE) image corresponding to one slice in the CEST volume with teal arrow pointing to the lesion (top), with average gray matter (GM), white matter (WM), and lesion segmentations overlaid (bottom). B. A four-pool Lorentzian model (magnetization transfer (MT), direct saturation (DS), nuclear Overhauser effect (NOE), and amide) was fit to the measured Z-spectrum (black). Average measured Z-spectra and fitted pools are shown for GM (left), WM (middle), and lesioned (right) tissue. C. Uncorrected average raw Z-spectra and inverted Z-spectra for the three tissue types (left). After subtracting the MT and NOE fitted pools, differences between GM and WM on the Z-spectra and inverted spectra upfield from water are less apparent for the amide contrast Z-spectra (middle). After subtracting the MT and amide fitted pools, differences between GM and WM on the Z-spectra and inverted spectra downfield from water are less apparent for the NOE contrast Z-spectra (right).