Lorenz, Anna; Sathe, Aditi; Zaras, Dimitrios; Yang, Yisu; Durant, Alaina; Kim, Michael E.; Gao, Chenyu; Newlin, Nancy R.; Ramadass, Karthik; Kanakaraj, Praitayini; Khairi, Nazirah Mohd; Li, Zhiyuan; Yao, Tianyuan; Huo, Yuankai; Dumitrescu, Logan; Shashikumar, Niranjana; Pechman, Kimberly R.; Jackson, Trevor Bryan; Workmeister, Abigail W.; Risacher, Shannon L.; Beason-Held, Lori L.; An, Yang; Arfanakis, Konstantinos; Erus, Guray; Davatzikos, Christos; Habes, Mohamad; Wang, Di; Tosun, Duygu; Toga, Arthur W.; Thompson, Paul M.; Mormino, Elizabeth C.; Zhang, Panpan; Schilling, Kurt; Albert, Marilyn; Kukull, Walter; Biber, Sarah A.; Landman, Bennett A.; Johnson, Sterling C.; Bendlin, Barbara; Schneider, Julie; Barnes, Lisa L.; Bennett, David A.; Jefferson, Angela L.; Resnick, Susan M.; Saykin, Andrew J.; Hohman, Timothy J.; Archer, Derek B. “The Effect of Alzheimer’s Disease Genetic Factors on Limbic White Matter Microstructure.” Alzheimer’s and Dementia 21, no. 4 (2025): e70130. https://doi.org/10.1002/alz.70130.
White matter in the brain helps different regions communicate effectively. As people age, and especially in conditions like Alzheimer’s disease, this white matter can deteriorate. Advanced brain imaging techniques, such as diffusion MRI, now allow researchers to study these changes in living individuals.
This study examined how genetic risk factors for Alzheimer’s disease may affect white matter in areas of the brain involved in memory—regions that are often affected early in the disease. Data from over 2,600 non-Hispanic White older adults, aged 50 to 101, were analyzed. Researchers looked at 36 genetic variants across 26 genes, assessed overall genetic risk using Alzheimer’s polygenic scores, and explored how these factors interacted with individuals’ cognitive status.
The analysis revealed that specific genes—TMEM106B, PTK2B, WNT3, and APOE—were linked to changes in white matter structure. Variants in the MS4A6A gene showed different effects depending on whether a person had cognitive impairment. Higher genetic risk for Alzheimer’s was also associated with increased free water in memory-related brain regions, which may signal early white matter damage.
These findings suggest that genes involved in brain development, fat metabolism, and inflammation may contribute to age-related changes in brain structure, particularly in people at higher genetic risk for Alzheimer’s disease.
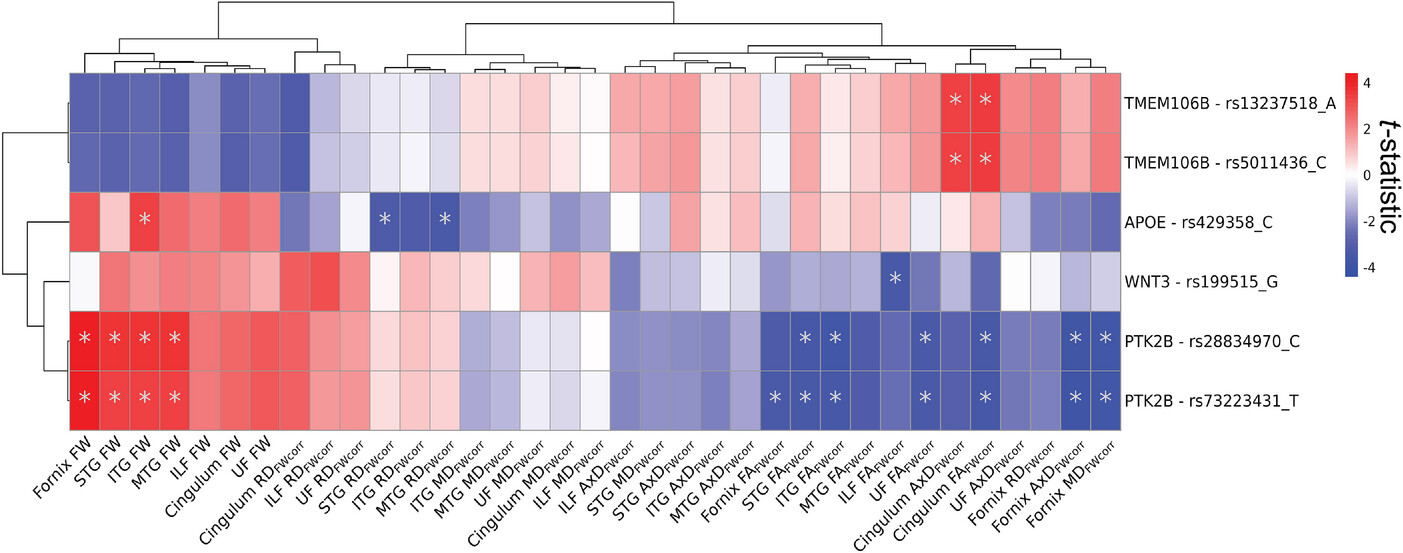
FIGURE 1
Effects of AD genetic variants on WM microstructure. The figure displays the t-statistics of the main effect AD genetic variant derived from linear regression models fitted separately to each WM microstructure of the limbic tracts measured by FW-corrected dMRI metrics (N = 35). Predictors were 36 genetic variants previously associated with AD and significant in UK Biobank NIDPs. Model: WM metric ≈ AD genetic variant + sex + age + PC1 + PC2 + PC3. The displayed results are filtered for genetic variants that displayed at least one FDR-significant association (pFDR < .05) with a dMRI metric. Asterisk indicates pFDR < .05. Associations were clustered with Euclidean distance approach using pheatmap R package (version 1.0.12). AD, Alzheimer’s disease; AxD, axial diffusivity; dMRI, diffusion magnetic resonance imaging; FA, fractional anisotropy; FDR, false discovery rate; FW, free water; ILF, inferior longitudinal fasciculus; ITG, inferior temporal gyrus transcallosal tract; MD, mean diffusivity; MTG, middle temporal gyrus transcallosal tract; NIDP, neuroimaging-derived phenotype; RD, radial diffusivity; STG, superior temporal gyrus transcallosal tract; WM, white matter.