Ali, Rosine; Alonga, Jules; Biampata, Jean-Luc; Kombozi Basika, Michael; Maljkovic Berry, Irina; Bisento, Nella; Blum, Emily; Bonnett, Tyler; Cone, Katherine; Crozier, Ian; Davey, Richard; Dilu, Ali; Dodd, Lori E.; Gulati, Iman; Hruby, Dennis; Ibanda, Augustin; Isse, Francis; Kasareka, Sylva Sivasingana; Kayembe, Gaby; Kojan, Richard; Luzolo, Esaie Kindombe; Lane, H. Clifford; Lawanga, Leader; Liesenborghs, Laurens; Shosongo Lunghe, Claude; Lula, Yves; Lusakibanza, Mariano; Lutete, Gaston Tona; Mbala-Kingebeni, Placide; Miranda, Alejandra; Mukadi-Bamuleka, Daniel; Mukendi, Gael; Lupola, Patrick Mutombo; Muyembe-Tamfum, Jean-Jacques; Ndungunu, Robin; Nganga, Bruce; Ntamabyaliro, Nsengi; Nussenblatt, Veronique; Omulepu, Imoite; Omalokoho Onosomba, John; Proschan, Michael; Rubenstein, Kevin; Saknite, Inga; Schechner, Adam; Shaw-Saliba, Kathryn; Sivahera, Billy; Smolskis, Mary; Tillman, Amy; Tkaczyk, Eric; Tshimanga, Celestin; Tshiani Mbaya, Olivier; Tshomba, Antoine; Yemba Unda Tshomba, Freddy; Vallee, David; Vogel, Susan; Weyers, Shera. “Tecovirimat for Clade I MPXV Infection in the Democratic Republic of Congo.” The New England Journal of Medicine 392, no. 15 (2025): 1484–1496. https://doi.org/10.1056/NEJMoa2412439.
Tecovirimat is a medication approved in the U.S. and Europe to treat mpox (formerly called monkeypox), based on animal studies and safety tests in healthy people. However, there hasn’t been clear proof from large, controlled studies in people who actually have mpox—until now.
In this study, researchers ran a carefully controlled clinical trial in the Democratic Republic of Congo (DRC) to test how well tecovirimat works and how safe it is for people infected with clade I mpox virus (MPXV), a version of the virus found in Central Africa. A total of 597 patients with mpox were randomly given either tecovirimat or a placebo (a lookalike pill with no active medicine), and everyone received supportive care.
The main thing the researchers looked at was how long it took for skin lesions (the sores caused by mpox) to heal. They found that people taking tecovirimat recovered in about 7 days, while those taking the placebo recovered in 8 days—a very small difference that was not statistically significant, meaning it could have happened by chance.
Other measures, like how quickly the virus disappeared from the body and how many people experienced side effects, were also similar between the two groups. About 1.7% of all patients died, which is lower than the 4.6% death ratereported for mpox in the DRC in 2023. Side effects occurred in about 71–73% of people in both groups, and serious side effects were rare.
In this study, tecovirimat did not significantly speed up recovery in patients with mpox caused by the clade I virus. However, it was found to be safe, with no major concerns about side effects. Further studies may still be needed to explore its use in different types of mpox or stages of illness.
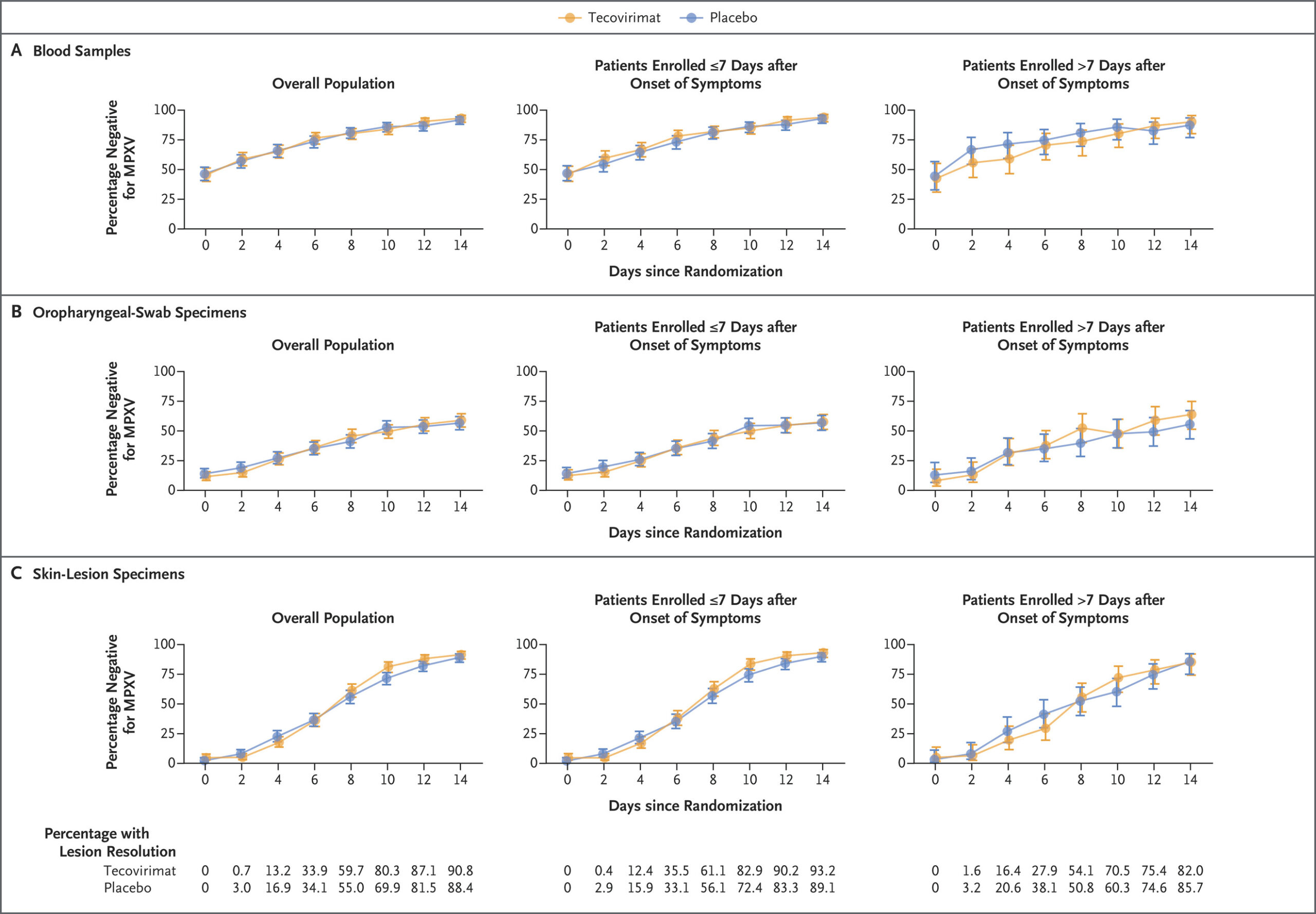
Fig 2.
Longitudinal PCR Results According to Trial Group and Symptom Onset in the Intention-to-Treat Population.
The percentage of patients testing negative for the virus that causes mpox (MPXV) by polymerase-chain-reaction (PCR) assays of blood samples (Panel A), oropharyngeal swab specimens (Panel B), and skin-lesion specimens (Panel C) is shown according to trial group and the timing of symptom onset (≤7 days vs. >7 days before randomization). Patients with a baseline positive or negative PCR result are included. The last-observation-carried-forward method was applied to valid PCR results from baseline to 14 days after randomization. Patients who have lesion resolution are not expected to have further positive PCR results for skin-lesion specimens, so patients who had lesion resolution on or before a given time point were assumed to be negative for MPXV if no valid PCR data were available. 𝙸 bars indicate 95% confidence intervals.