Li, Jing; Jacobse, Justin; Pilat, Jennifer M.; Kaur, Harsimran; Gu, Weihong; Kang, Seung Woo; Rusznak, Mark; Huang, Hsin-I; Barrera, Julio; Oloo, Pauline A.; Roland, Joseph T.; Hawkins, Caroline V.; Pahnke, Andrew P.; Khalil, Marian; Washington, M.Kay; Wilson, Keith T.; Williams, Christopher S.; Peebles, R.Stokes, Jr.; Konnikova, Liza; Choksi, Yash A.; Hammer, Gianna Elena; Lau, Ken S.; Goettel, Jeremy A. “Interleukin-10 production by innate lymphoid cells restricts intestinal inflammation in mice.” Mucosal Immunology (2025). https://doi.org/10.1016/j.mucimm.2025.02.005.
Interleukin-10 (IL-10) is a protein that helps regulate the immune system and is especially important for keeping the gut’s immune environment balanced. Many types of immune cells make IL-10, but for it to work properly in the gut, it needs to send signals to certain immune cells called CX3CR1+ mononuclear phagocytes. Without this, mice can develop gut inflammation (colitis) on their own.
In this study, we used special glowing markers and precise targeting methods to study how IL-10 works in the gut during inflammation triggered by a chemical called anti-CD40. We found that a type of immune cell called innate lymphoid cells (ILCs), specifically those that have a gene called Rorc, produce IL-10 when there is gut inflammation.
When we deleted the Il10 gene only in these Rorc-expressing ILCs, it changed the behavior of other gut immune cells (macrophages) and made gut inflammation worse in multiple models of colitis. The IL-10-producing ILCs had features of both ILC2 and ILC3 cells, and most of the ILC3s had a subtype called NCR+. We also saw that a signal molecule called Ccl26 was found more in IL-10+ ILCs, but much less in ILC3s that didn’t have IL-10. Because CCL26 can interact with CX3CR1, we used a detailed imaging method and saw more ILCs located near CX3CR1+ cells during gut inflammation.
Finally, we created special mice that help track RORγt+ cells (a group that includes ILC3s) and showed that transferring these cells into mice that couldn’t make IL-10 in ILC3s could reduce disease symptoms and normalize macrophage behavior.
In summary, this study shows that IL-10 made by a group of ILCs plays a key role in keeping the gut’s immune system balanced, likely by directly affecting gut macrophages.
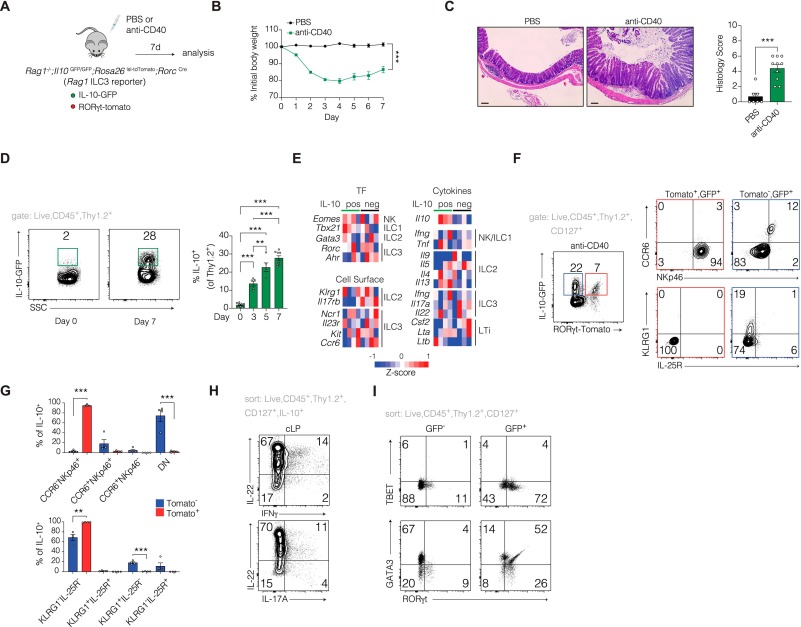
Fig. 1 IL-10 is induced in ILCs during intestinal inflammation. A) Schematic of anti-CD40 colitis model in Rag1-/-;Il10GFP;Rosa26lsl-tdTomato;RorcCre mice. B) Time course of body weight change following anti-CD40 treatment. C) Hematoxylin and Eosin (H&E)-stained colonic sections (left) and inflammation score quantified by a pathologist blinded to genotype and treatment (right). (n ≥ 7), scale bar = 100 μm. D) Representative flow cytometric analysis of GFP expressing cells gated on Live,CD45+,Thy1.2+ at day 0 and 7 days following anti-CD40 treatment (left) with longitudinal assessment of IL-10-GFP expressing cells (right) (n ≥ 5). E) Heatmap showing normalized expression of differentially expressed genes between GFP+ and GFP– ILCs sorted from the colons of Rag1-/-;Il10GFP;Rosa26lsl-tdTomato;RorcCre mice 7 days post-anti-CD40 treatment, n = 4 mice per group. F) Representative flow cytometric analysis of IL-10-GFP expression on colon ILCs 7 days post anti-CD40 (left) assessed for expression of ILC3 markers (CCR6, NKp46) and ILC2 markers (KLRG1, IL-25R) (right) and quantified in G. H) IL-10-GFP+ ILCs sorted from the colon 7 days post-anti-CD40 treatment were pooled from 5 mice and restimulated with PMA/ionomycin for 4 h in the presence of a Golgi inhibitor. Cells were then stained for intracellular IL-22, IFNγ, and IL-17A. I) Flow cytometry plot depicting GFP+ and GFP– ILCs that were sorted from pooled colons of 5 mice 7 days post-anti-CD40 treatment and stained for nuclear expression of RORγt, TBET, and GATA3. Data are pooled from 2 or more independent experiments. Each dot represents an individual mouse. Bars are the mean ± SEM. **P < 0.01, ***P < 0.001. one-way ANOVA: A; unpaired t-test: C,D,G.