2022 Trainees



|
Hannah Bhakta
Mentors: Rachana Tomar, Michael Stone |
|
|||
|
"Replication Bypass of N-(2-Deoxy-D-erythro-pentofuranosyl)-urea Adducts by Human DNA Polymerase η" DNA can be damaged by many natural sources, and can lead to cancer, neurological diseases, and aging. N-(2-Deoxy-D-erythro-pentofuranosyl)-urea adducts are formed by oxidative damage done to guanine or thymine. Human DNA polymerase η (“hpol η”) is known to aid in error-free replication when the duplex contains a damaged nucleotide. We made the urea-adducted 12-mer deoxyoligonucleotide sequence from a thymine glycol-containing template and annealed it with a 5’-FAM-labeled primer sequence. Next, we performed replication assays with purified hpol η after incubating with the urea-modified DNA duplex and verified the correct nucleotide insertion opposite the modified base. In this preliminary study of urea adducts, we used gel electrophoresis in an effort to confirm this insertion of the dNTP opposite the urea lesion. Bio. Hannah Bhakta is a rising junior at Valparaiso University majoring in biochemistry and minoring in Spanish. At her home university, she works under Dr. Jeffrey Pruet to synthesize Argemone mexicana-inspired antimicrobial products, and she has been working on this project for three semesters now. Bhakta has presented at the Indiana Academy of Science as well as colloquiums and symposiums at Valparaiso University. She is also a recipient of the Edith S. Lessor Memorial Scholarship for excellence as a woman in the field of biochemistry. At Vanderbilt University through the Chemical Biology REU, she has pursued structural biology research in the Stone Lab to further understand the capabilities of human DNA polymerase η. |
|
|
|||

|
Yingrong Chen
Mentors: Benjamin Brown, Jens Meiler |
|
|
||
|
"Developing Structure-based Drug Discovery Methods at the BCL-Rosetta interface" Computational modeling of protein-small molecule interactions can be used to study biological activities and facilitate small molecule drug discovery. Rosetta is a software package primarily used for macromolecular structure simulation and structured-based drug design. In contrast, the Biochemical Library (BCL) is a ligand-based chemoinformatic toolkit focusing on small molecules. Integration of BCL into Rosetta enables simultaneous manipulation of protein pockets and small molecules, and, therefore, allows researchers to develop more complex drug discovery protocols that are currently unavailable in the computer-aided drug discovery (CADD) field.However, the current BCL-Rosetta interface is limited to performing structure-based chemical structure perturbations. Thus, this project aims to expand the BCL -Rosetta integration to enable additional tasks, such as small molecule alignment, chemical property prediction, and neural network-based binding energy estimation. Utility methods are developed to convert between Rosetta Pose and BCL FragmentComplete objects. Then, BCL applications are wrapped into Rosetta Movers and SimpleMetrics to enable modular access. Ongoing effort in this project will lead to the development of state-of-the-art protocols for small molecule drug design, such as methods for free energy perturbation. In the long term, this project will enable more accurate protein-small molecule interaction modeling in biological research and broaden the scope of computer-aided drug discovery to satisfy unmet research demands. Bio. Yingrong Chen (also goes by Momo) is a rising junior from Emory University majoring in Chemistry and Computer Science. She is passionate about developing and applying computational methods to chemical and biomedical problems. This summer, she works on computer-aided drug discovery software with Benjamin Brown at the Meiler Lab. Back at Emory, she has also worked with protein dynamics in Alzheimer’s Disease and molecular dynamics simulation of psoriasin protein structure. |
|
|
|||

|
Hunter Davis
Mentor: Allison Walker |
|
|
||
|
"Computationally Guided Antibiotic Discovery from Natural Products of Streptomyces" As more pathogens become resistant to current antibiotics, there is an increasing need for new antibiotics. Traditionally this was done by high throughput screening of natural products, but this method alone has become uneconomical. As a result, the number of antibiotics discovered per year has decreased. In response to this issue, one strategy is to use computational means to identify active metabolites. Such methods include the use of: antiSMASH to predict biosynthetic gene clusters, machine learning for activity prediction, GNPS to identify analogs through molecular networking, and MZmine for comparison of mass spectra to known compounds. The goal of this study is to validate these methods on Streptomyces noursei and Streptomyces yunnanensis. This genus is of interest due to its reputation as a producer of bioactive metabolites. Solid and liquid cultures of the bacteria were extracted and screened for antibacterial activity against Bacillus subtilis. There is evidence to support the presence of up to four novel antibacterial compounds. The mass spectral data do not provide known matches when compared to multiple databases. Despite the lack of structural data, a list of potential masses can be produced for one of the compounds while an incomplete peptide sequence can be made for another. The results of this study demonstrate that it is possible to fulfil the need for new antibiotics more efficiently with a computationally guided approach. Bio. Hunter Davis is a rising senior at Tennessee Technological University majoring in biochemistry & cell and molecular biology. He began his exposure to the field of biochemistry during high school with an internship focused on the development of monoclonal antibodies. His most significant research experience as an undergraduate at TTU has been the involvement with topoisomerase inhibition towards cancer therapy in the Jiang Lab. |
|
|
|||

|
Madeline Herwig
Mentors: Eli McDonald, Minsoo Kim, Lars Plate |
|
|
||
|
"Deletion of Translational Slippery Sites in CFTR Changes Expression Levels in a Mutation Dependent Manner" Slippery sites are specific nucleotide sequences in mRNA that affect the rate of ribosomal translation. These sites cause the ribosome to “slip”, resulting in a frameshift in the reading frame of the ribosome. These sites have recently been discovered in the transcript of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) the causative mutated protein in Cystic Fibrosis (CF) a lethal, monogenetic disease. CFTR is a membrane protein expressed in epithelial cells. It is an anion channel protein that is responsible for the transport of chloride and sodium ions. The most common mutation of CFTR is the deletion of phenylalanine 508 (F508del) and 90% of CF patients have at least one allele containing this mutation. Notably, slowing translation speed increases F508del expression. However, the relevance of these identified slippery sites in CFTR and their effect on its translation is largely unknown. Here, we used affinity purification mass spectrometry to quantify protein interactors between wildtype CFTR and F508del with and without these slippery sites conserved. We hypothesize that there will be differences in interactors between the conserved slippery site and deleted slippery site. Bio. Madeline Herwig grew up in Geneva, Illinois and is a rising senior at Augustana College studying Biochemistry and Public Health. Throughout her undergrad, she has worked in Dr. Pamela J. Trotter’s lab investigating the biochemical relevance of glutamine dehydrogenase in Yarrowia Lipolytica, a nonconventional yeast model. After completing her undergraduate studies, she hopes to continue her education and pursue a PhD in Chemical Biology. Outside of the lab, Madeline enjoys playing her violin, singing, and spending time with her family and friends. |
|
|
|||
|
Alec Jones
Mentor: Nathan Schley |
|
|
|||
|
|
|
|
|||

|
Cale Locicero
Mentors: Kalinskar Bera, Matthew Knowe, Jeffrey Johnston |
|
|
||
|
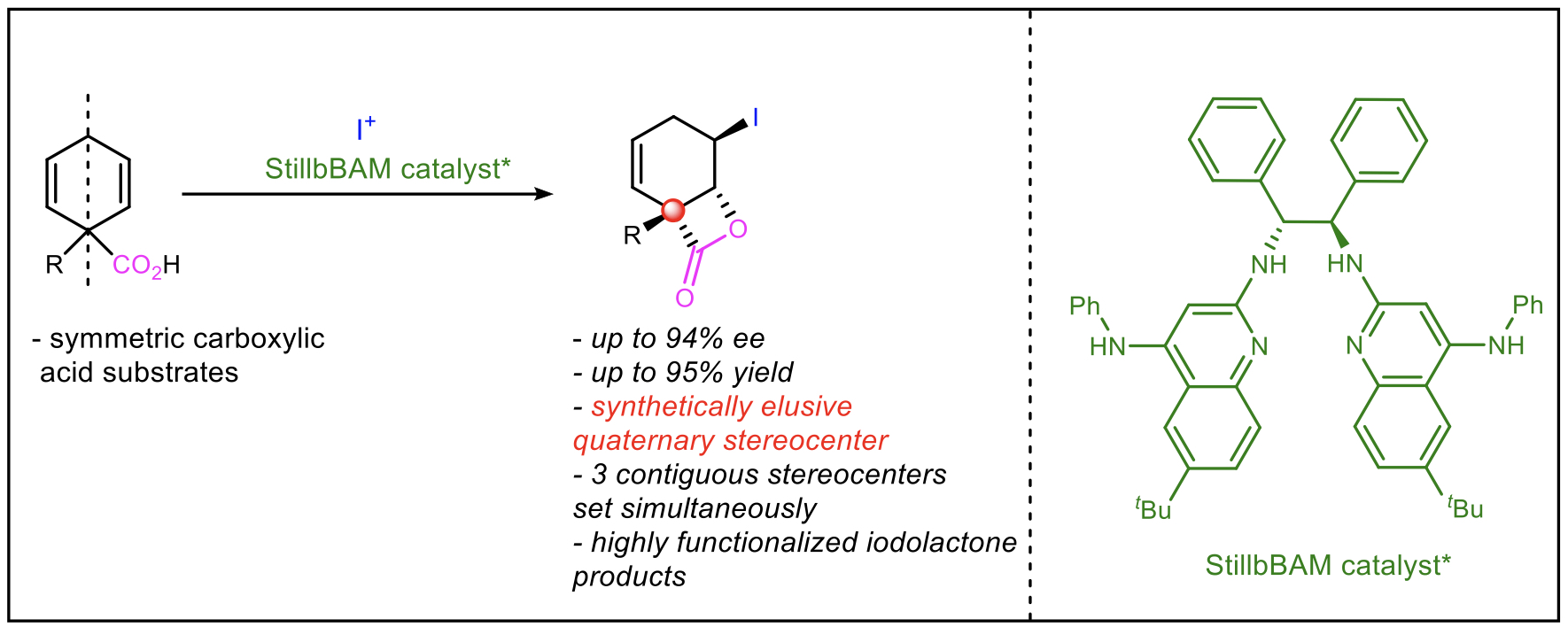
"Enantioselective Desymmetrizations of Carboxylic Acids Using Chiral Proton Catalysis"
Within the field of asymmetric catalysis, desymmetrization reactions present themselves as valuable opportunities to build stereochemical complexity from simple prochiral starting materials. Despite the utility of these reactions, desymmetrizing halolactonizations are few, perhaps due to the difficulties associated with controlling the nucleophilicity of the carboxylate and activation of the halonium ion while achieving group-selective (Z-alkene) differentiation. Herein we report the enantioselective desymmetrization of cyclohexadiene carboxylic acids using chiral proton catalysis. The resulting β-lactones contain an all-carbon quaternary stereocenter and are formed in high selectivity.
The reaction products are purified by chromatography, structurally characterized using spectroscopic methods, and analyzed for enantiomeric enrichment using chiral HPLC. The ultimate goal of this endeavor is to advance the limits of organocatalysis by addressing a synthetically challenging chemical transformation that can access stereochemically complex building blocks for applications toward syntheses of biologically and pharmaceutically relevant small molecules. Bio. Cale Locicero is an Astronaut, Goldwater, and American Chemical Society Scholar and is an Ogden Honors College Senior pursuing a B.S. in chemistry at Louisiana State University. He is President of the Student Affiliates of the American Chemical Society and Vice President of Faculty Relations of the LSU Research Ambassadors. Cale is a research assistant in the Kartika Group, where he develops novel organic reactions for the production of medicinally relevant chemical structures with special emphasis on the creation of all-carbon quaternary stereocenters. Additionally, Cale is a 2022 NSF-REU student in the Johnston Lab in the Vanderbilt Chemical Biology REU Program. He performs research in enantioselective chiral proton catalysis with a focus on alkene desymmetrization reactions. From September 2020 to February 2021, he performed research under Professor Adam T. Melvin in the LSU Cain Department of Engineering and created microfluidic concentration gradient generators to test synergistic combinations of antibiotics against E. coli biofilms. Cale has experience in nuclear magnetic resonance spectroscopy, infrared spectroscopy, gas chromatography-mass spectrometry, chromatographic techniques, microfluidics, soft lithography, 3D printing, bacterial culture, and computer programming. In his free time, he enjoys reading scientific literature on various topics, hiking, and programming. |
|
|
|||
|
|
|
|
|||

|
Myaisha Lucas
Mentors: Elizabeth Adams, Steven Townsend |
|
|
||
|
"Synthesis of an AAT Donor to Enable Access to Bacterial Glycans" Bacterial exopolysaccharides possessing zwitterionic charge motifs (ZPSs) are known to modulate the cellular immune system. AAT (2-acetamido-4-amino-2,4,6-trideoxy-D-glucopyranose) is a rare deoxy-amino sugar previously found within the repeating unit of ZPSs isolated from several different bacteria. Recently, a ZPS was isolated from P. vulgaris and is composed of AAT and two D-ribitol phosphate (D-Rib-ol-5-P) residues. Thus, we initiated a synthesis campaign to target the O-polysaccharide repeating unit to elucidate its role in immune modulation.Bio. A rising senior from Raleigh, North Carolina, Myaisha, is majoring in Biochemistry at High Point University. At her home institution in North Carolina, her undergraduate research focuses on the synthesis of biologically active compounds. She is a student instructor for organic chemistry and co-founder of a student ambassador program aimed at prospective students interested in natural sciences. In addition to being the Vice President of the American Chemical Society student chapter on her campus, she is involved in nearly every instrumental ensemble on her campus. The clarinet player plans to attain a Ph.D. in chemistry and work on a total synthesis project. As for her career, she ultimately hopes to work in drug development for the pharmaceutical industry. |
|
|
|||
|
|
|
|
|||

|
Esabella Powers
Mentors: Pragun Tuladhar, David Cliffel |
|
|
||
|
"Microfluidic Electrochemical Biosensors for Simultaneous Multianalyte Detection" Monitoring electrolytes within biological fluids can provide vital information about homeostasis and cellular metabolism. Electrolytes are typically analyzed from urine, blood, sweat, etc. using solid contact electrodes. However, current techniques do not allow for continuous monitoring, low volume measurements, and multianalyte detection. In this work, a screen-printed electrode (SPE)-based potentiometric sensor for potassium ion detection under laminar flow was developed. An ion selective membrane was added to the platinum working electrodes of an 8-channel SPE enclosed in a microfluidic chamber. Ion selective membrane formulation, conducting polymer layer, and electrode conditioning parameters were optimized for linear range, sensitivity, and selectivity. Potentiometric data was collected by a CHI 1440 potentiometer. The potassium sensor gave a linear response between 58 μM–25 mM KCl in phosphate buffered solution and had a sensitivity of 71.88 ± 5 mV decade-1. A poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonate) (PEDOT: PSS) conducting polymer layer was investigated to enhance selectivity in the presence of other cations. The potassium sensor will ultimately be a part of a combined potentiometric and amperometric detection platform capable of simultaneously measuring dynamic concentrations of ions and small molecules. In the future, this sensor will be used for continuous monitoring of various biomarkers from tissue cultures to assess the progression of disease or toxicity.
|
|
|
|||
|
|
|
|
|||

|
Irina Samsonova
Mentors: Wook Shin, John Yang
Home Institution: University of Missouri
|
|
|
||
|
"Benchmarking Configurational Entropy of Small Organic Molecules: Maximal Entropy Approach" Free energy computation is vital to computational modeling of proteins and protein-ligand interactions for drug design and discovery. The accurate computation of entropy, i.e., the central component of free energy, presents a big challenge to the community. This work develops an approach for calculating the configurational entropy of small organic molecules and benchmarks computational prediction against experimental observation. Conformational entropy is calculated using the Maximum Information Spanning Tree (MIST) approach with the molecular topology determined by the maximal entropy method. Translational, rotational, and momentum terms are added to the computed configurational entropy for comparison with the experimental values. This work is a preliminary benchmark and can be further developed to treat macromolecular systems, such as peptides, proteins, or a protein-ligand system. As the next step, we will also employ a deep generative model to enable entropy calculations for QM systems. These studies will lay the foundation for entropy-based functional molecule design and discovery. Bio. Irina Samsonova is a rising Junior at the University of Missouri. She is currently working on completing a Bachelor of Science in Chemistry with ACS Certification as well as a B.S. in Physics and a Certificate in Computational Physics. Irina has worked in computational chemistry at Mizzou for 2 years and has also worked as a teaching assistant for 1 year. After completing undergraduate studies, she plans on attending Graduate school for Physics and pursuing theoretical and computational Physics. |
|
|
|||
|
|
|
|
|||

|
Christina Troll
Mentors: Alexandra Blee, Walter Chazin |
|
|
||
|
"Investigation of the XPA-RPA Interaction in Nucleotide Excision Repair" Nucleotide excision repair (NER) is an essential process by which cells repair bulky DNA lesions. NER requires the action of multiple proteins, including both Xeroderma Pigmentosum Complementation Group A (XPA) and Replication Protein A (RPA). XPA and RPA both interact with the DNA as well as with one another via two interacting regions with differing affinities. While there are various mutations in XPA that can cause lower NER activity, the mechanism remains unknown. Several NER-deficient mutations of interest are located in one of the RPA-interacting regions of XPA. This work seeks to test the hypothesis that XPA mutation reduces interaction with RPA and causes NER defects. Recombinant wild type XPA protein as well as the XPA mutants M113I and D114Y were expressed and purified. Isothermal Titration Calorimetry (ITC) was performed to test the binding affinity of purified XPA and RPA. Using this technique, the dissociation constant (KD) was determined. By comparing the KD values of XPA mutants to the KD value of wild type protein, it was possible to test whether the XPA mutants have altered RPA binding affinity. Preliminary analyses have revealed that single point mutations are not sufficient to significantly alter the XPA-RPA binding affinity, which suggests that the reduced NER activity associated with these XPA mutants should be attributed to another factor. Bio. Christina Troll is a rising senior biochemistry major at the University of Notre Dame in Notre Dame, Indiana with minors in bioengineering and real estate. There, she performs biochemical research under the guidance of Dr. Katharine White. She studies the consequences of increased intracellular pH (pHi) on the polarization of the Golgi apparatus and subsequently, single cell migration using the light activatable optogenetic tool Archaerhodopsin. This fall, Christina will be presenting this research at the American Chemical Society conference. In addition to research, she seeks to aid other students by working as an undergraduate teaching assistant and is involved with the Chemistry/Biochemistry Club and the Graduate School Student Club. She made Dean’s List in the Fall of 2021 and will graduate with Honors upon completion of her thesis this spring. |
|
|
|||
|
|
|
|