Exceptions to the Octet Rule
Odd-Electron Molecules: Electrons in a Lewis structure occur in pairs (bonding pairs or lone pairs). Molecules that have an odd number of electrons can not satisfy the octet rule on all of their atoms. In drawing the Lewis structure of such a molecule, leave an unpaired lone electron on one of the atoms and minimize the separation of formal charge. Example
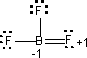
Octet-Deficient Molecules: Some compounds do not have sufficient electrons for each atom to achieve an octet consistent with other constraints on the molecule. Covalent compounds of beryllium and boron are frequently octet deficient. Example
Valence-Shell Expansion: Third-row and heavier elements can exceed the octet rule by using their empty valence d orbitals. More than eight electrons are allowed around the central atom. This is called Valence-Shell expansion. Example
![]()