Examples for Valence-Shell Expansion Molecules
Sulfur Hexafluoride SF6
SF6 has 48 valence electrons. Use 12 electrons to form the SF bonds and satisfy the octet-rule for fluorine (fluorine always follows the octet-rule). With this, we have used up 48 electrons, but sulfur has 12 electrons around it.
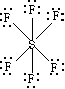
Sulfur has empty 3d orbitals. The extra 4 electrons go into the formerly empty 3d orbitals.
![]()