Example of Octet-Deficient Molecules
Boron trifluoride BF3
But BF3 reacts very energetically with molecules such as water and ammonia that have available electron pairs (lone pairs). This indicates that BF3 is electron-deficient. Experimental evidence also indicates there is no double bond in BF3 (fluorine never forms double bonds.) Furthermore, a positive formal charge on F is extremely disfavored. So placing only six electrons around the boron is more correct. At least it assigns zero formal charge to all atoms.
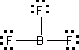
![]()