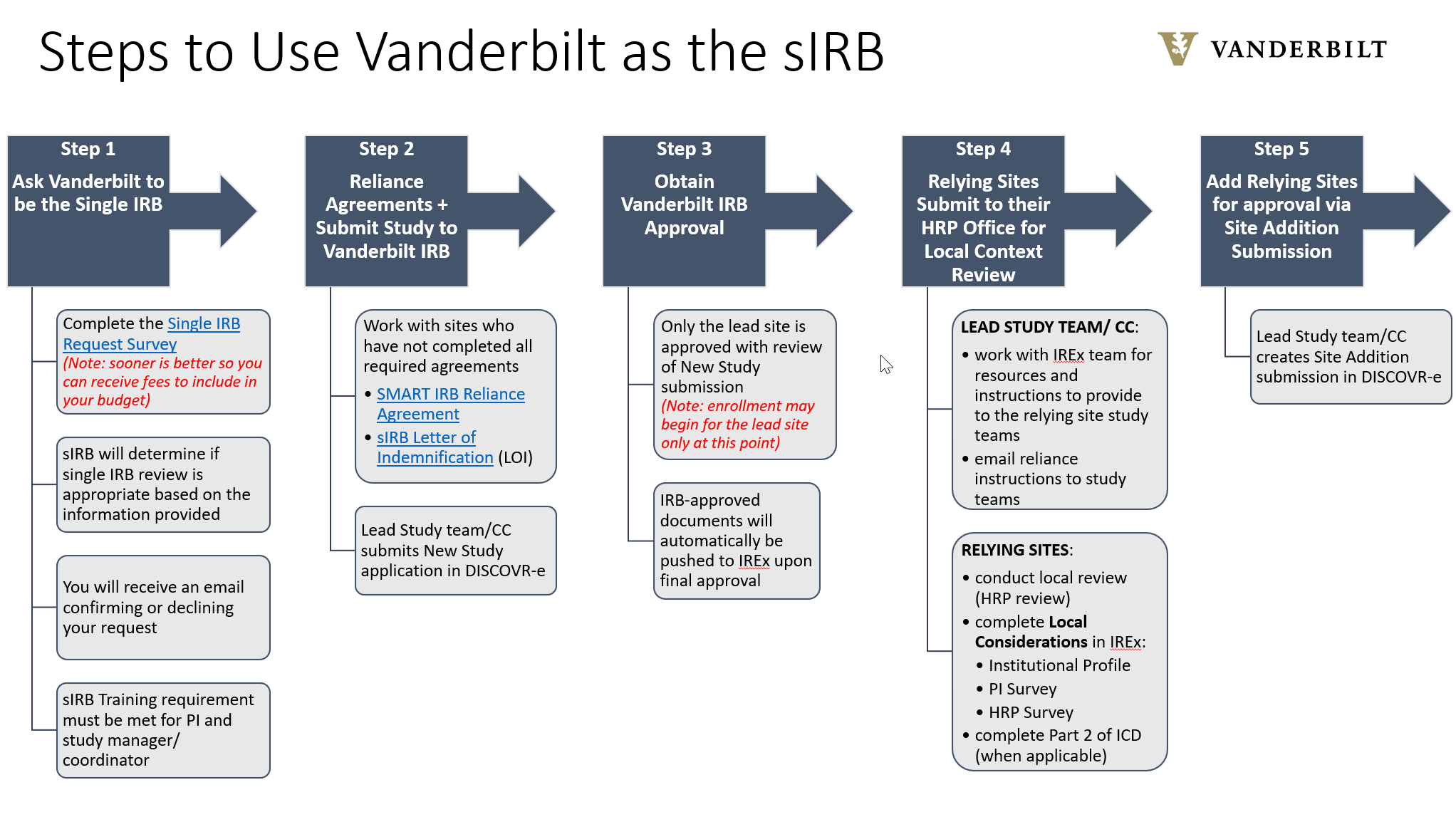
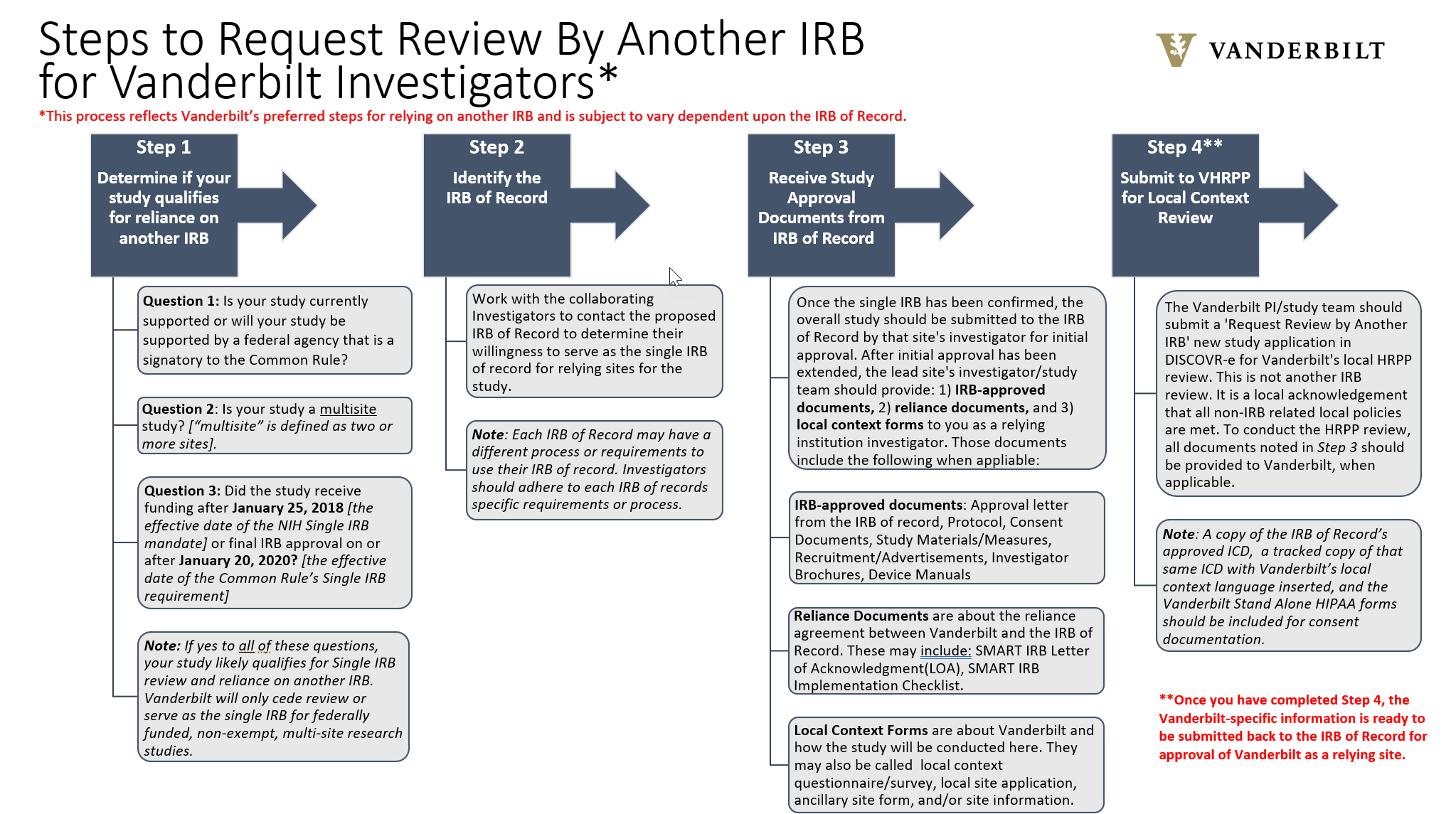
- Vanderbilt requires completion of a Single IRB Request survey in order to determine whether or not a study qualifies for Single IRB review.
- Use the orange tabs below to complete the Single IRB Request.
- When asking Vanderbilt to be the Single IRB, allow a response time of up to 5 business days.
- Click on the overview images below each tab to enlarge for readability.
Currently, VHRPP will only cede review or serve as the single IRB for federally funded research studies in order to comply with the Single IRB mandate. If you have any questions about single IRB review, please contact Kayley Pratt at kayley.pratt@vumc.org
-
HIPAA Authorization Form - Ceded Studies Only
-
Single IRB Submission Tip Sheet When Vanderbilt is the SIRB - for ALL lead study teams
Local Information - for Vanderbilt teams
Table