Spectrophotometry
Some molecules absorb light, and as a result, solutions containing these molecules appear as "having color." Analytical chemists use the properties of light absorption to determine the concentration of a compound present in solution.
The concentration of the solution is determined through the use of a spectrophotometer. The spectrophotometer produces a beam of monochromatic light with an initial radiant power, Po. When a sample of the solution is placed into the path of the beam, its molecules absorb a portion of the light and result in the reduction of the radiant power, P. A solution having a high concentration of a light absorbing molecule will absorb more light than a dilute solution of the same molecule.
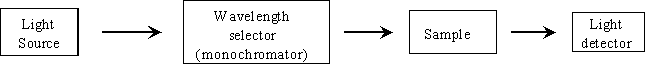
![]()