MicroPhysTech - Microfluidic Physiological Technologies for in vitro instrumentation and control of single cells and tissues
Innovative Systems Biology and Systems Engineering at Vanderbilt
VIIBRE, the Vanderbilt Institute for Integrative Biosystems Research and Education, was created in 2001 with a $5 million, five-year grant from the Vanderbilt Academic Venture Capital Fund to foster and enhance interdisciplinary research in innovative systems biology and systems engineering at Vanderbilt, integrated with a strong focus on undergraduate, graduate, and postdoctoral education.
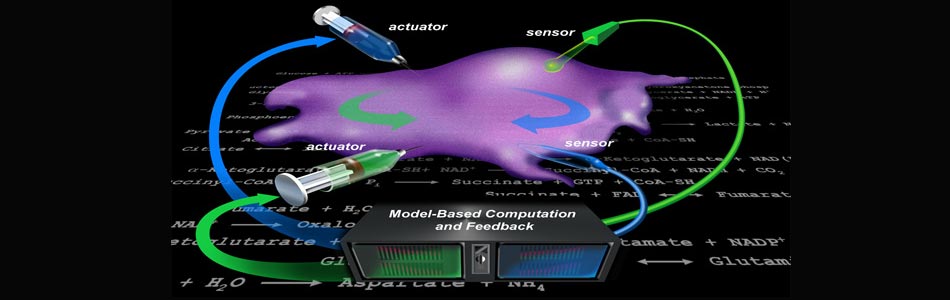
VIIBRE's mission is to invent the tools and develop the skills that are required to conduct research in systems biology - in other words, to elucidate the multitude of intracellular metabolic and signaling control mechanisms by which biological systems regulate homeostasis, differentiation, development, growth, repair, and responses to physiological and environmental stimuli. Control of cellular function is ubiquitous among living systems and occurs over spatiotemporal scales that range from rapid local control of transient molecular signaling events to slower regulatory mechanisms governing biological activity throughout an entire organism over its lifespan. This spatiotemporal breadth presents measurement and modeling challenges that require a coordinated, collaborative effort by scientists, engineers, and physicians whose expertise spans multiple disciplines.
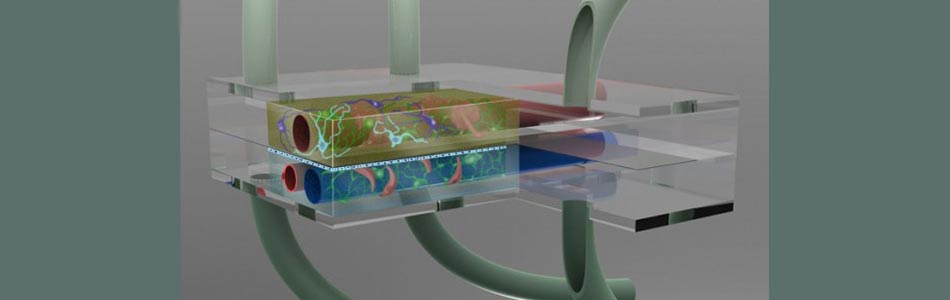
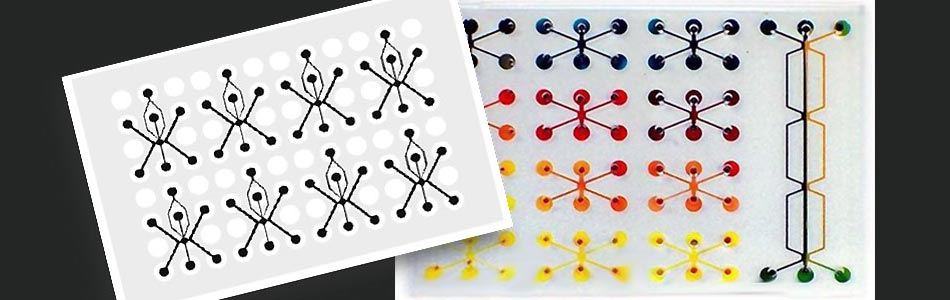
Taking advantage of Vanderbilt's existing strengths in science, medicine, engineering, and education, VIIBRE has instituted a broad program in BioMicroElectroMechanical systems ( BioMEMS ) and advanced analytical and optical instrumentation and software to address pressing questions in systems biology. VIIBRE's activities are carried out in on- and off-campus collaborations in research areas such as cellular biosensors, bioprocess controllers, mathematical models for wound healing and cancer, infectious disease detection, biomedical imaging, and cellular/tissue engineering. One of VIIBRE's long-term challenges is to design and build hybrid silicon/biological systems (microfluidic cell habitats) that propose and generate models and conduct experiments on themselves to identify the principles (represented by mathematical equations) that describe cellular metabolic and signaling dynamics.