Home » Supported Research » Clinical validation and testing of percutaneous cochlear implantation
Clinical validation and testing of percutaneous cochlear implantation
Title: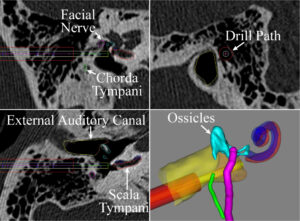
Clinical validation and testing of percutaneous cochlear implantation
Project Number:
5R01DC008408-13
Contact PI/Project Leader:
Robert Labadie
Award Organization:
National Institutes of Health
Abstract:
The objective of this proposal is to perform a clinical feasibility study using our previously developed approach for minimally invasive cochlear implantation (CI), thereby bringing the approach closer to widespread clinical adoption. This translational work is the culmination of years of research and, if successful, will fundamentally change CI surgery and enable more patients to receive an implant. Clinical significance comes from the large number of patients who could benefit from a CI but have not received one, in part due to the complexity and invasiveness of the procedure. Despite their effectiveness, less than 10% of CI candidates have received an implant. The reasons for the underutilization include high procedure costs and a shortage of otolaryngologists trained to perform CI surgery. To bridge this gap, technological improvements to lower the cost of the procedure and make it easier to perform are necessary. Our approach to reduce the invasiveness and simplify the surgery uses image-guided surgery along with novel image processing algorithms. This approach replaces manual removal of a large volume of bone with a narrow drill path from the skull surface to the cochlea automatically aligned along the path using a patient-specific stereotactic frame. This approach may reduce the cost of the procedure and enable less specialized surgeons to perform the surgery. The technical and clinical innovations in this proposal include: (1) clinical translation of a novel surgical approach for which we have recently received an Investigation Device Exemption from the FDA, (2) multi- layered, redundant safety checks based on patient specific models and intra-operative hardware verification, (3) testing of an insertion tool with custom insertion depths based on patient specific anatomy, and (4) enabling individuals with ossified cochleae, a consequence of meningitis, to receive CI with this approach because current surgical techniques result in suboptimal electrode placement and performance in this group. Our approach consists of three Specific Aims. In Aim 1 we will complete an FDA approved clinical feasibility study using our minimally invasive technique comparing several key metrics between minimally invasive CI and traditional CI (electrode placement, audiological outcomes, and time of intervention). Aim 2 will further improve minimally-invasive CI through the development and testing of a custom CI insertion tool capable of inserting straight and pre-curved electrode arrays within the constrained workspace of the minimally invasive tunnel to the cochlea. We will perform a clinical assessment of the tool and compare insertions to those with traditional tools. In Aim 3 we will adapt the surgical approach to patients with ossified cochleae, a.k.a. labyrinthitis ossificans, a consequence of meningitis in which the cochlear duct becomes obstructed by new bone formation. To do this we will modify the minimally invasive technique to drill out multiple channels surrounding the auditory nerve endings and use post-operative imaging to specify how the electrodes stimulate the nerve.
