Coming soon!
The A. B. Hancock Jr. Memorial Laboratory is a transformational leader in recruiting world-class scientists, mentoring innovative talent, and developing the collaborations and infrastructure needed to excel in the challenging field of cancer research. Engaged in both basic and translational research, the Hancock lab seeks new insights into the causes of cancer and works toward the development of innovative approaches to early detection, prevention, and treatment. The lab's unifying focus is the complex relationship between inflammation and cancer. Chronic inflammation can cause DNA damage, induce genetic mutations, and stimulate cell growth that leads to cancer. Inflammation that forms in response to a developing tumor can foster an environment that promotes growth and spread of the cancer. Hancock Laboratory investigators are using state-of-the-art techniques to map the earliest changes in inflammation-induced cancer in order to identify new targets for early detection. Their exploration of the role inflammation plays during cancer development, growth, and spread is guiding efforts towards better ways to prevent and treat cancer. The Laboratory has made many seminal contributions that have been or are being translated into the clinic. For example:
- Hancock Laboratory investigators published the first report that identified, in normal human DNA, a form of damage caused by inflammation. They subsequently showed that this DNA damage causes the same type of genetic mutations that are detected in cancer, a revelation that was hailed as the "smoking gun" linking inflammation to cancer.
- The discovery that individuals who regularly take aspirin have lower colon cancer mortality prompted Hancock Lab Director, Dr. Larry Marnett, to assemble a team of Vanderbilt scientists and physicians to explore the molecular basis for the aspirin effect. This team included Drs. Raymond DuBois, Jason Morrow, Jack Roberts, Robert Coffey, and Dr. Marnett. Research by the Hancock team revealed that aspirin lowers the rate of progression of premalignant cells to malignant ones in the colon by inhibiting the production of inflammatory hormonal substances called prostaglandins. Aspirin blocks the action of an enzyme called cyclooxygenase (abbreviated COX) that carries out the first step in prostaglandin synthesis. Although prostaglandins have many beneficial effects in the body, they can also promote the development of cancer. Blocking prostaglandin production with aspirin helps to lower the incidence of cancers of the colon and other organs, as well.
- The Hancock team discovered that a new form of the COX protein (called COX-2) is expressed in premalignant and malignant colon tumors but not in surrounding normal tissue. This selectivity of expression makes COX-2 an ideal target for cancer detection, prevention, and treatment. These discoveries led to clinical trials showing that the COX-2 inhibitor celecoxib (Celebrex) is an effective cancer preventive agent and a useful adjuvant cancer therapeutic agent.
- Hancock Laboratory investigators developed the first imaging agent that can detect COX-2 in inflammation and cancer. This provides an important tool for early detection of cancer, definition of surgical margins, and evaluation of response to therapy. It is particularly impressive for the detection of very early lesions that are not visualized by simple microscopic analysis. This technology is being adapted for clinical use.
- The Hancock Laboratory assembled a team of investigators led by Drs. Alex Brown and Craig Lindsley to target another key enzyme of inflammation and cancer growth called phospholipase D. The successful outcome of this effort is the discovery of powerful phospholipase D inhibitors that show promise as treatments for brain cancer.
- Dr. Marnett has used the resources of the Hancock Laboratory and the Vanderbilt Institute for Chemical Biology (VICB), which he also directs, to assemble the infrastructure for drug discovery at Vanderbilt. This includes the construction of a high-throughput screening facility to identify biologically active compounds, a chemical synthesis facility to synthesize improved drug candidates, and an antibody facility to prepare antibodies for detection and treatment of cancer. Through a partnership with the Vanderbilt Ingram Cancer Center (VICC), the VICB recruited Dr. Stephen Fesik, Vice-President of Cancer Therapeutics at Abbott Laboratories, to lead Vanderbilt's Cancer Drug Discovery effort. Dr. Fesik's laboratory is rapidly making inroads in the discovery of promising new therapeutic agents directed against cancer targets that were previously thought to be "undruggable".
- Mutations cause cancer, and the identities of these mutations are being determined comprehensively through The Cancer Genome Atlas program supported by the National Cancer Institute. This will eventually allow the individual fingerprint of every human cancer to be established. A critical question is how to use this information to develop drugs against those cancers. Dr. Marnett secured major funding from the National Cancer Institute to support a multi-disciplinary team that is using genetic information to identify new molecular targets for drug discovery. The Hancock team, including Drs. Carlos Arteaga, David Cortez, Stephen Fesik, Jennifer Pietenpol, David Weaver, and Dr. Marnett, is focusing on triple-negative breast cancer — the deadliest form of breast cancer. Their efforts have identified multiple new targets for breast cancer drug development and have discovered some promising candidate drugs with which to attack those targets.
Hancock Laboratory investigators have won numerous awards for their innovative work. The scientific achievements nourished in the Hancock Lab have led Vanderbilt-Ingram researchers to distinction in major national leadership positions. Included among these key leaders are:
- Larry Marnett, Ph.D., is the Mary Geddes Stahlman Professor of Cancer Research, Director of the Hancock Lab, and Director of the Vanderbilt Institute of Chemical Biology. He is a Fellow of the American Association for the Advancement of Science and a Fellow of the American Chemical Society. He served for 25 years as the founding editor of Chemical Research in Toxicology. The American Chemical Society's Division of Chemical Toxicology honored Marnett with its first Founders' Award in 2008 to recognize his contributions to the field of chemical toxicology. In particular, this award honored his seminal study of how molecules that are produced during inflammation can damage DNA, leading to cancer. More recently, Marnett was chosen to receive the first 2012 George and Christine Sosnovsky Award from the American Chemical Society for chemical research on cancer leading to improved therapy.
Marnett was the founding Chair of the Chemistry in Cancer Research group for the American Association of Cancer Research. He is the author of more than 450 research publications and 14 patents.
- Jennifer Pietenpol, Ph.D. is the director of the Vanderbilt-Ingram Cancer Center and the B.F. Byrd Jr. Professor of Molecular Oncology. She is one of eight members on the National Cancer Advisory Board, which advises the secretary of the U.S. Department of Health and Human Services and the National Cancer Institute director. She is also on the board of directors of the American Association for Cancer Research and the Susan G. Komen for the Cure Scientific Advisory Committee. Susan G. Komen for the Cure is the largest source of breast cancer research funding outside of the U.S. government.
Pietenpol's research laboratory is engaged in work on biochemical pathways that control processes of tumor suppression, development, metabolism, and aging. Pietenpol and her colleagues are examining the role of the p53 family signaling axis in normal growth and tumor development of cells, with a special focus on a hard-to-treat form of breast cancer known as triple negative breast cancer. In 2012, she was awarded one of only five global innovation grants from the GE Healthymagination Cancer Challenge.
- Robert J. Coffey Jr., M.D., John B. Wallace Professor of Medicine, Ingram Professor of Cancer Research, Professor of Cell and Developmental Biology, and a Fellow of the American Association for the Advancement of Science. Coffey serves as Director of Vanderbilt-Ingram’s Special Program of Research Excellence (SPORE) in gastrointestinal cancer. In the competition for its current funding, this Vanderbilt-Ingram SPORE received the highest score in the country from the National Cancer Institute. One of only six in the nation, the GI SPORE focuses on innovative, translational research to reduce and prevent colorectal cancers, a leading cause of cancer deaths in both men and women.
Coffey’s research is devoted to understanding the molecular events responsible for the development of gastrointestinal cancer and using that knowledge to improve detection and therapy. His investigations on the role of epidermal growth factor (EGF) signaling in tumorigenesis has led to innovative approaches to the treatment of gastronintestinal cancers. Recently, he extended his research to focus on a debilitating premalignant stomach disorder known as Ménétrier’s disease. Ground breaking treatment of a patient with this condition using drugs that block EGF signaling led to an ongoing clinical trial with promising results. Recently, Coffey established a partnership with GE Global Research that will explore GE’s cancer mapping technology to investigate the role of intestinal stem cells in tumor formation. This work promises to provide unprecedented insight into the processes of tumor initiation, progression, and metastasis.
- Carlos Arteaga, M.D., holds the Donna S. Hall Chair in Breast Cancer. He is Director of the Vanderbilt-Ingram Breast Cancer Program, the Center for Cancer Targeted Therapies, and the Vanderbilt Ingram Cancer Center Research Network. He leads the Vanderbilt-Ingram Specialized Program of Research Excellence (SPORE) in breast cancer. His research team is examining oncogene signaling in breast tumor cells and working on the development of molecular therapies for human breast cancer. He serves on the Susan G. Komen for the Cure Scientific Advisory Committee with Dr. Pietenpol.
Arteaga is a member of one of the international cancer research “Dream Teams” organized and funded by Stand Up To Cancer (SU2C), a charitable initiative of The Entertainment Industry Foundation created to accelerate cancer research that will get new therapies to patients quickly and save lives. His research is supported by grants from the National Institutes of Health, the American Cancer Society, the Lee Jeans Translational Breast Cancer Research Program, the Robert J. Kleberg, Jr. and Helen C. Kleberg Center for Personalized Cancer Medicine, and a Dream Team Translational Research Grant.
- Stephen W. Fesik, Ph.D., Orrin H. Ingram II Professor in Cancer Research, is the new leader for a cancer drug discovery program developed jointly by the Vanderbilt Institute of Chemical Biology and the Vanderbilt-Ingram Cancer Center. He is a Fellow of the American Association for the Advancement of Science and the author of more than 240 research articles and 10 patents. He holds a prestigious National Institutes of Health Pioneer Award for his boldly innovative research in drug discovery using fragment-based approaches and structure-based drug design. Prior to coming to Vanderbilt, Fesik was divisional vice president for cancer research at Abbott Laboratories, where he developed a pipeline of compounds that showed promising anticancer activity in early stage clinical trials. In 2012, he received the AACR Award for Outstanding Achievement in Chemistry in Cancer Research from the American Association for Cancer Research, and in 2010, he received the SBS Technology Innovation Award from the Society for Biomolecular Sciences.
Fesik is developing new approaches for targeting proteins currently considered “undruggable.” One of his projects targets K-Ras, a protein that is mutated in 90 percent of pancreatic cancer patients.
- Craig Lindsley, Ph.D., Professor of Pharmacology and Chemistry, holds the William K. Warren Jr. Chair in Medicine. Lindsley serves as the Co-Director of the Vanderbilt Center in Neuroscience Drug Discovery and Principal Investigator for the Vanderbilt Specialized Chemistry Center for Accelerated Probe Development, supported by the National Institutes of Health (NIH) Molecular Libraries Roadmap. He also currently serves as founding editor of ACS Chemical Neuroscience.
The American Society for Pharmacology and Experimental Therapeutics (ASPET) honored Lindsley with a 2010 ASPET Astellas Award in Translational Pharmacology. The award recognizes pharmacologic research that bridges basic science and translational applications that improve human health. The novel approaches that he pioneered at Merck, prior to coming to Vanderbilt, changed the approach to medicinal chemistry throughout the company and had a major impact on virtually every therapeutic area represented by the company. More recently, he was awarded the 2013 Philip S. Portoghese Lectureship, from the Journal of Medicinal Chemistry and the Division of Medicinal Chemistry of the American Chemical Society.
What Do We Know About the Causes of Cancer, and How Do We Know It?
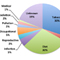
Considering the recent major advances in our understanding of cancer biology, it may seem surprising that we still have questions concerning what actually causes cancer. To be sure, we have great certainty regarding some cancer causes (carcinogens), as in the case of cigarette smoking. However, considerable confusion surrounds a much larger number of suspected causes about which the evidence is conflicting. For example, the most complete and authoritative source regarding the causes of human cancer is the International Agency for Research on Cancer (IARC), an organization that brings together scientists from many disciplines to identify human carcinogens so that preventive measures can be planned and adopted. Since 1971, the IARC has extensively evaluated over 900 agents as potential human carcinogens (complete list available at the IARC website). The definition of “agent” is very broad and includes individual chemicals, complex mixtures, physical agents (such as radiation), occupational exposures, biological agents (such as viruses and bacteria), and lifestyle factors (such as smoking and diet) (Figure 1). Of the 900 agents evaluated, only 108 have definitively been classified as carcinogenic to humans, while 508 remain in a category for which there is insufficient evidence to make any reasonable judgment. To understand the source of this confusion and to better evaluate the risk of cancer from exposure to a particular suspected agent requires some understanding of how we determine the causes of cancer in humans.
The IARC uses four sources of information to evaluate an agent as a human carcinogen. These are:
- Exposure data: An agent cannot be a significant cause of cancer in the human population unless people are exposed to it. Thus, the IARC evaluates when the agent first appeared in the human population, how it is used, how many people come into contact with it, and whether its use is increasing or decreasing over time.
- Studies of cancer in humans: Obviously, moral, ethical, and practical considerations prevent direct experimentation with potential carcinogens in people. Consequently, scientists must rely on epidemiology to obtain information regarding the causes of human cancer. Epidemiology studies the patterns of health and disease in large populations, typically using one of three basic approaches.
- Comparison studies investigate large populations in different geographic regions or cultures that display markedly different rates of the disease of interest. A search of data concerning lifestyles and the environment of the two populations provides clues as to the basis for the observed differences (Figure 2).
- In case-control studies, the investigators identify a group of patients with the disease of interest (cases) and match them to a group of healthy people (controls) who are similar with regard to factors such as age, sex, socioeconomic status, and region of the country. Detailed medical information about both groups reveals key differences that may provide clues as to the cause of the disease in the cases.
- In cohort studies, a large number of people (cohort) are enlisted and asked to provide detailed information about their lifestyle and health status over time. Then, as individuals in the cohort become ill with a disease of interest, the accumulated information is used to look for potential causes.
- Studies of cancer in animals: Although animal experimentation has been increasingly criticized on ethical grounds, it remains the best source of information regarding the effects of a carcinogenic agent in an intact organism similar to man. Typically, rats or mice (Figure 3) are exposed to high doses of the suspected agent for an extended period of time (usually up to two years), and investigators evaluate the animals regularly for the appearance of cancer. These experiments are time-consuming and expensive, and there is no guarantee that the animals will respond to the agent the same as a human. Furthermore, since human cancer usually develops from exposure to much lower doses of an agent over a longer period (ten to twenty years), animal experiments can not faithfully replicate this process. Despite these limitations, all known human carcinogens that have been studied adequately have been shown to be carcinogenic in at least one animal species. Thus, data from animal experiments continue to be a valuable source of information.
- Mechanistic studies: We now have a better understanding of the processes by which a normal cell becomes a cancer cell. For example, we know that the DNA, which contains the blueprint that controls all cellular functions, is badly damaged in cancer cells. We also know that factors that promote cell division and inflammation favor the development of cancer. This information allows investigators to conduct experiments at the cellular or chemical level to determine exactly how an agent might act as a carcinogen (Figure 4). For example, the discovery that an agent directly damages DNA would lend strong support to the conclusion that it causes cancer, though that information alone would not be proof that the agent is carcinogenic to humans.
When evaluating an agent as a human carcinogen, the IARC assembles all available information from these four sources and makes a judgement based on the accumulated evidence. Unfortunately, the sources of information are frequently incomplete and may be contradictory. As noted above, epidemiology and animal studies carry with them some basic flaws. Hence, the result of the evaluation has so frequently been inconclusive. As a result, the IARC classifies agents that it evaluates into one of five groups, according to the certainty of the available data:
- Group 1: The agent causes cancer in humans. These are agents for which the combined evidence is so strong that there is no serious doubt that they cause cancer in humans. There are currently 108 Group 1 agents.
- Group 2A: The agent probably causes cancer in humans. These are agents for which the combined evidence is strong, but some doubt remains. For example, there may be convincing evidence of carcinogenicity in animals, and it is clear how the agent could cause cancer, but data in humans are lacking. There are currently 64 Group 2A agents.
- Group 2B: The agent possibly causes cancer in humans. These are agents for which there is enough evidence that they may cause cancer in humans to raise a concern, but significant doubt remains. There are currently 272 Group 2B agents.
- Group 3: The agent can not be classified. For these agents, there is insufficient data to draw any conclusions. There are currently 508 Group 3 agents.
- Group 4: The agent probably does not cause cancer in humans. These are agents for which the preponderance of evidence indicates that they are not carcinogenic in humans. There is currently 1 Group 4 agent.
It may seem surprising that the number of agents in Group 4 is so low. To understand why, it is important to note that the IARC only investigates an agent if there is some reason to suspect that it is carcinogenic. Thus, the vast majority of substances that we encounter in every day life are considered harmless, so they have not been reviewed.
Two familiar agents reviewed by the IARC provide an excellent illustration of how the evaluation process works (Figure 7). The first, tobacco smoking, is the single major avoidable cause of human cancer in the world today. The second, exposure to magnetic and electric fields from power lines and other sources, has received considerable attention in the popular media but remains a source of controversy. Using IARC monograph Volume 83 (published in 2004) Tobacco Smoke and Involuntary Smoking and IARC monograph Volume 80 (published in 2002) Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields, we will explore how the IARC reached its classification, placing tobacco smoke in Group 1 and Electric and Magnetic Fields in Groups 3 and 2B, respectively.
Tobacco Smoking - Overwhelming Evidence of Human Carcinogenicity
Exposure to tobacco: Although human use of tobacco dates back thousands of years in the western hemisphere, worldwide spread did not begin until October of 1492, when Christopher Columbus received tobacco from the Arawak natives of the West Indies. By 1600, tobacco had been introduced to countries throughout Europe, and to Persia, India, Japan, China, and regions of Africa. Despite this rapid dissemination, however, the modern widespread practice of tobacco smoking was delayed until 1880, when James Bonsack patented the first machine to manufacture cigarettes. The first 50 years of the 20th century saw the rapid adoption of cigarette smoking in industrialized countries, and cigarettes remain the primary means of tobacco consumption today. In nearly all cultures, increases in smoking have occurred first among men with use by women increasing later and usually to a lesser degree. The overall consumption of cigarettes in the United States peaked in 1965, coincident with the first Surgeon General’s report on the hazards of smoking (Figure 8). However, increasing use of cigarettes in developing countries has kept global consumption on the rise. Today, there are 1.2 billion smokers worldwide, representing 29% of the population over 15 years of age.
Studies of cancer in humans: Early indications of the link between smoking and cancer came from the rapid increase in lung cancer that occurred in the early to mid 20th century. This increase paralleled the increase in cigarette smoking, though it was delayed by about 20 years, the time required to develop cancer in humans (Figure 9). We now have large quantities of data that tie smoking to human cancer, and most of these data come from large population studies (epidemiology). Interpretation of data from this kind of study can be challenging. Although it is usually not hard to classify a person as a current smoker, former smoker, or nonsmoker, difficulties may arise in quantifying how much a person has smoked, particularly over the course of many years. Investigators must also be concerned about the reliability of information provided on surveys typically used in large population studies. In addition, results may be complicated by factors that affect cancer rate but are not directly related to smoking. Examples include socioeconomic status, diet, local environment, occupational exposures, etc. Despite these challenges, the large number of smokers in the population and the long period of time over which studies have been conducted combine to increase the reliability of the data. The IARC evaluated the results of 45 large population (cohort) studies, from around the world, nearly all of which involved tens of thousands of people. The results of these studies were reinforced by an even larger number of case-control studies. The conclusions were clear. Smoking is the single major cause of the most common form of human cancer (lung cancer), which afflicts 1.2 million people worldwide each year.
The data clearly show a strong association between how long a person has smoked and his/her risk for lung cancer. For example, the risk of being diagnosed with lung cancer is 100-fold higher for people who have smoked for 45 years as compared to those who have smoked for only 15 years. These results indicate that the full effect of smoking in a population will not be evident until 50 years after the practice becomes common. Fortunately, stopping smoking helps to reverse the increased cancer risk, although the benefit takes time to develop. Thus, the sooner a person stops, the greater the risk reduction will be later in life. There is also some benefit from the use of lower tar, filter cigarettes; however the magnitude of this effect observed in different studies has varied from 0 to 50%. The lifetime probability that a smoker will develop lung cancer depends on his/her specific smoking practices, personal metabolic makeup, and risk of dying from other causes. In general, the risk is estimated to be 24% in men and 11% in women by age 85, though the risk of dying from all cigarette-related diseases is as high as 50%.
Studies of cancer in animals: Early attempts to demonstrate that smoking causes cancer in animals were hampered by variations in susceptibility to cancer among different animal species and the difficulty of exposing animals to cigarette smoke in a way that mimics smoking by humans. However, studies in hamsters, rats, mice, rabbits, and dogs have now all shown a link between smoking and cancer. Animal studies have also shown that inhaling cigarette smoke increases the carcinogenic potency of asbestos, radon, plutonium, and some chemicals. Administration of tobacco smoke condensates on the skin of animals causes cancer and increases the potency of other skin carcinogens.
Studies of how smoking can cause cancer: Cigarette smoke contains thousands of compounds, many of which are toxic. The IARC has reviewed the carcinogenicity of some of the compounds in cigarette smoke on an individual basis. As a result, we know that smoke contains at least 11 Group 1, 11 Group 2A, and 40 Group 2B compounds. We also know that at least 6 tobacco smoke carcinogens react chemically with DNA. This means that they interact with the components of DNA in such a way that the structures of both molecules are altered. Most often, a piece of the carcinogenic molecule becomes attached to the DNA forming a kind of damage known as a DNA adduct (Figure 10). If this damage is not repaired before the cell replicates its DNA and divides, the result can be a misreading of the DNA code, leading to a permanent mutation. DNA adducts have been detected in the lungs and other tissues of smokers. Lung cancers that result from cigarette smoking contain unique patterns of mutations, and many of these mutations alter the control of critical cellular functions such as growth and division, survival, and cell-to-cell interactions. These are exactly the functions that are abnormal in cancer cells.
In addition to causing DNA damage, smoking injures the cells lining the airways of the lungs, reducing their ability to remove toxic particles (Figure 11). Components of smoke affect cells’ ability to inactivate drugs and toxic compounds, leading to changes in the way these compounds are cleared from the body. Smoking-related damage triggers inflammation in the lung, which leads to an environment that can foster the survival of cancer cells. Thus, smoking is capable of acting both at the level of a cell’s DNA and at the level of the cell’s environment to promote the development of cancer.
Summary: It is clear from all four criteria used by the IARC that tobacco smoking is not only carcinogenic, but it is carcinogenic in humans. A large number of humans are, by their own choice, heavily exposed to cigarette smoke. Data from numerous epidemiology studies indicate an association between smoking and cancer in humans. Although we emphasized lung cancer here, the IARC found convincing evidence that smoking increases the risk for numerous other cancers, including cancer of the oral cavity, naso- oro- and hypopharynx, nasal cavity, paranasal sinuses, larynx, esophagus, stomach, pancreas, liver, kidney, ureter, urinary bladder, uterine cervix, and bone marrow. In addition, smoke inhalation causes cancer in animals, and it is easy to explain how smoking can lead to cancer based on studies of the components in smoke and their effects on cells. Together, these data strongly support the Group 1 designation for cigarette smoking.
Electric and Magnetic Fields - Many More Questions than Answers
Exposure to electric and magnetic fields: The IARC evaluated the possible carcinogenicity of two kinds of electric and magnetic fields. Fields that remain constant in strength over time are designated static fields. Alternatively, a field’s strength may fluctuate with a regular pattern, most commonly at a low frequency. These are designated extremely low frequency (ELF) electric and magnetic fields. Examples include those that are generated by the alternating current used in our electric power system and all of the equipment and appliances operated by that system.
We are all constantly exposed to electric and magnetic fields from both natural and manmade sources (Figure 12). The earth provides a static magnetic field of 25 to 65 microTeslas (μT). (The unit of measure for magnetic fields, the Tesla, is based on the force that the field exerts on a charged particle of standard size). In addition, we are exposed to many ELF fields, most of which are due to manmade sources. Usually, environmental exposures to such fields are quite low, with electric fields typically in the range of 5 to 50 volts/meter (V/m) and magnetic fields from 0.01 to 0.2 μT. However, much higher exposures can occur for brief periods of time, for example, through the use of an electric appliance, such as a hair dryer or electric blanket, that is operated close to the body.
The study of the health effects of electric and magnetic fields has been greatly hampered by the difficulty in assessing exposure levels. We now have the instruments to accurately measure fields in various locations, but it is not clear exactly where and how such measurements should be taken. Fields vary in different parts of a building and can change with time due to the use of appliances. People often move from one environment to another with very different field levels during the course of a day. Since it is impractical to directly measure fields over long periods of times in large numbers of environments, field strengths are often calculated from the presence of a source, such as a high voltage power line, using the distance from the source and the strength of the field it generates. However, these calculations may be subject to considerable error. Adding to the difficulty is the fact that the development of cancer in humans occurs over the course of many years, so assessing home or workplace field levels at any one period of time may not reflect all of the risks to which an individual has been exposed. Furthermore, the highest fields to which most people are exposed are usually only two- or three-fold higher than average levels, so differences in exposure are not large. Together, these factors combine to make the evaluation of risk from static and ELF electric and magnetic fields a challenge.
Studies of cancer in humans: The concern that ELF magnetic fields may be carcinogenic in humans began with a study published in 1979, which reported that children living close to high voltage power lines were at increased risk for developing childhood leukemia. Since that time, numerous additional studies have been conducted in an attempt to determine if there is, indeed, a risk and if so, the magnitude of that risk. The IARC panel evaluated the results of one cohort study and 18 individual case-control studies. The panel also reviewed the results of two separate pooled analyses of multiple studies. Although these studies revealed conflicting results, a consistent trend emerged suggesting that exposure of children to magnetic fields of greater than 0.3 μT or 0.4 μT were at 1.7-fold or 2.0-fold risk of contracting leukemia, respectively. The IARC concluded that these findings were not likely due to chance. However, they also noted that low response rates to study surveys and the difficulty of accurately assessing magnetic field strength for the large number of homes needed for these studies may have led to systematic errors that could have affected the results.
A large number of other studies have attempted to determine if electric or magnetic fields cause cancer. These include assessments of risks from power lines for other forms of childhood cancer, risks for childhood and adult cancer from the use of certain appliances (water beds, electric blankets, televisions, hair dryers, sewing machines), and risks from occupational exposures in adults. Although some of these studies have suggested associations between electric or magnetic fields and certain forms of cancer, the overall results have been inconsistent and have failed to demonstrate an increasing effect with increasing dose. The IARC noted that many, if not all, of these studies suffered from the problems of accurately assessing exposures and controlling for other factors, such as cigarette smoking or exposure to carcinogenic chemicals.
Studies of cancer in animals: Under experimental conditions, it is possible to expose animals or cells in culture to electric fields as high as 150 kV/m and magnetic fields as high as 2,000 µT. These are a thousand-fold higher than those typically experienced by humans in daily life, but high levels are necessary in laboratory experiments due to the much shorter duration of the studies (up to two years), as compared to the time it takes for human cancer to develop (10 to 20 years).
The IARC evaluated four long-term studies of the effects of ELF magnetic fields in animals (three in rats and one in mice). In three of the studies, there was no association between exposure to high level magnetic fields and the development of over forty different kinds of cancer. In the fourth study, an association between magnetic field exposure and a form of thyroid cancer in male rats only was seen. Other studies have evaluated the effects of electric and magnetic fields on the ability of known carcinogens to cause cancer in animals. As in the case of the human studies, some have suggested that field exposure increases the potency of other carcinogens, but the results have been inconsistent.
Studies of how electric and magnetic fields can cause cancer: The attempt to evaluate the carcinogenicity of electric or magnetic fields is hampered by the lack of understanding of how they could cause or promote the development of cancer. Static magnetic fields exert forces on moving charged particles, orient magnetic structures (think of a compass needle), and affect the energy levels of some molecules. However, these effects of static magnetic fields do not penetrate deeply beneath the surface of the human body. Exposure to ELF electric and magnetic fields induces electric currents in tissues. These can be as high as 1 mV/m under conditions of some occupational exposures, but residential exposures are much lower. Furthermore, we do not know how the presence of electric currents, even strong ones, in tissues can lead to cancer.
The IARC evaluated a large number of studies that showed no adverse effects of electric or magnetic fields on the reproductive, immune, hematologic (blood), nervous, or endocrine (hormonal) systems in humans or animals. A number of studies indicated that ELF magnetic fields affect the secretion of melatonin, which is produced in the brain in coordination with the day/night cycle. However, once again, conflicting data have been reported. Some studies show that exposure of cells in culture to very high electric or magnetic fields leads to DNA damage, changes in cell growth and division, and cell death. However, it is unclear how the effects observed at these very high field strengths are relevant to exposures of people in their day-to-day lives.
Summary: Based on the apparently consistent finding that exposure to ELF magnetic fields is associated with increased risk of leukemia in children, the IARC designated this type of field as a possible human carcinogen (Group 2B). For the other types of fields, the lack of consistent evidence of carcinogenicity in humans or animals and the lack of an understanding of how they might be carcinogenic led the IARC to place them in Group 3 (agents that can not be currently classified).
Frequently the results of studies, such as the one published in 1979 showing an association between ELF magnetic fields and childhood leukemia, are publicized in the popular media. Such results understandably raise concern in the general public, especially among those who may be exposed. However, in cases such as ELF magnetic fields, where exposures are generally low and difficult to measure, and the increased risk is also low (compare 2-fold for ELF magnetic fields to 100-fold for cigarette smoking), the publication of conflicting results is highly likely. This leads to considerable public confusion and a lack of confidence in science. For this reason, it is important to remember that the assessment of cancer risk in humans is a difficult, time-consuming job, especially in the case of relatively weak carcinogens, and conflicting, often contradictory results are to be expected. Although the potential risks of such agents should not be completely ignored, we are more likely to benefit by focusing our greatest concern towards reducing or eliminating exposure to strong carcinogens, such as cigarette smoking, asbestos, and ultraviolet light (sunlight), for which the data are clear and protective action can be taken.
On December 23, 1971, then President Richard Nixon signed the National Cancer Act, which marked the beginning of what has become known as the “war on cancer”. Considering the fact that just two years previously, the United States put a man on the moon, finding a cure for cancer appeared to be a reasonable and attainable goal. Why, then, after more than 40 years of intensive research, does that goal remain elusive?
To be sure, the past four decades have witnessed tremendous strides, particularly in our understanding of cancer biology. Especially important is the discovery of the role of DNA mutations in the development of cancer. In the nucleus of every human cell (with the exception of eggs and sperm), there are 46 chromosomes made up of long molecules of DNA (Figure 1). These chromosomes carry the blueprint that dictates every aspect of the cell’s structure and function. Although cells have many mechanisms to protect their DNA and/or repair it, damage can occur, usually through exposure to a limited number of chemicals or high energy radiation. If not repaired, this damage can lead to a permanent change in the DNA’s structure, a mutation, which will then be passed on to the daughter cells when the cell divides. It has long been clear that mutations play a role in causing cancer, but only recently have we come to appreciate exactly how mutations lead to the abnormalities observed in a malignant cell.
One of the most astounding discoveries in recent years is that cancer cells harbor not just one or two mutations, but thousands (Figure 2). Considering the fact that a single mutation that leads to malfunction of a critical cellular process can be fatal, it is hard to imagine that cells carrying such massive damage to their genetic blueprint manage to survive. Even more amazing is that this accumulated damage leads cancer cells to:
- divide and grow rapidly in an out-of-control fashion
- stimulate the growth of local blood vessels to provide needed nutrition
- break free of the normal constraints of tissue structure, allowing them, eventually, to spread to other regions of the body
- appear to be immortal, continuing to survive under conditions that would normally lead to cell death
Recent detailed studies of the mutations in cancer cells show that they lead to abnormalities in cellular functions that control growth and division, stimulation of blood vessel growth, cell-to-cell interactions, and even death. In other words, the abnormal behavior of cancer cells is, in many cases, easily explained on the basis of their damaged DNA blueprint. In addition, most cancer cells harbor mutations that impair their ability to repair their DNA, which helps to explain how they accumulate so much damage.
We have known for many years that cancers differ depending on their site of origin (i.e., breast cancer behaves differently from lung cancer). This is explained, in part, by distinct groups of mutations typically found in the cancers in each organ site. However, outside of these common characteristics, we have now come to realize that the overall pattern of mutations is unique for each individual patient’s tumor, and that they change with time.
This leads to the important conclusion that cancer is not a single disease, or even a group of diseases. In fact, there are as many different cancers as there are cancer patients. An important outcome of this discovery is the understanding that we will likely never find a single cure for cancer. However, this does not mean that the quest is hopeless. The identification of specific mutations in cancer has led to new insight into ways that malignant cells can be killed or controlled. Furthermore, our newfound ability to identify key mutations in individual cancers is allowing us to design therapy that is most appropriate for each patient. Much work remains to fully realize this “personalized” approach to cancer therapy, but early successes with the newest “smart drugs” offer hope for more effective treatment with fewer side effects, and ultimately, even a cure.
Despite great strides in understanding and combatting cancer in recent decades, a cure for many forms of cancer, especially in the case of advanced disease, remains an elusive goal. Furthermore, most cancer therapies are expensive and associated with significant adverse side effects. Thus, prevention remains the single best weapon in the fight against this devastating disease, leading quite naturally to an important question. Just how many cases of cancer can actually be prevented?
The answer to this question comes primarily from epidemiology, the study of disease in large human populations. An excellent article by Doll and Peto, published in 1981 (Journal of the National Cancer Institute, Volume 66, pages 1192-1308) provides a comprehensive overview of the ways that we estimate the number of preventable cancer cases. More recent articles [Montesano and Hall (2001) European Journal of Cancer, Volume 37, pages S67-S87 and Adami et al. (2001) European Journal of Cancer, Volume 37, pages S118-S127] provide updates based on ongoing research, but their conclusions remain essentially the same as those of Doll and Peto. Here, we summarize the key points outlined by these authors.
Multiple lines of evidence contribute to the assessment of the number of cases of cancer that are preventable. These include:
- Differences in the occurrence of cancer between communities. Worldwide epidemiological studies show striking differences in the incidence of almost all kinds of cancer between different regions of the world. From the lowest- to highest-risk regions, these differences can be as much as 100-fold. Examples include a 100-fold difference in the rate of liver cancer between England (low incidence) and Mozambique (high incidence), a 35-fold difference in the rate of lung cancer between Nigeria (low incidence) and England (high incidence), and a 300-fold difference in the rate of esophageal cancer between Nigeria (low incidence) and Iran (high incidence). Simply the fact that places exist where incidences of a particular form of cancer are very low suggests that the higher incidence rates in other parts of the world are preventable.
- The change in cancer incidence rates that occur when people migrate. Usually, when groups of people migrate from a region of low to high (or high to low) incidence of any type of cancer, their risk for that form of cancer becomes similar to that of their new location. Sometimes this takes several generations to occur, but the fact that people exhibit the cancer risk that is associated with their location rather than their ethnicity suggests that the differences in risk are due to environmental factors rather than genetics. Examples include cancer incidence rates among black Americans, which more closely resemble those of white Americans than black populations in Africa (Figure 2), and rates among Japanese residents in Hawaii, which more closely resemble those of white populations in Hawaii than Japanese populations in Japan.
- Changes in cancer incidence over time. These data must be interpreted carefully, because factors such as improving diagnostic techniques and an aging population can affect cancer incidence statistics. However, time trends such as the increase in lung cancer with the increase in cigarette smoking and decrease in stomach cancer with better food preservation methods (Figure 3) help to illustrate the role of environmental and life style factors in causing cancer.
- Reduction in exposure to a suspected cause of cancer that leads to reduced cancer rates. This is, perhaps, the best evidence that cancer can be prevented. Examples include the decrease in the incidence of lung cancer in the developed world that has resulted from a decrease in cigarette smoking that began in the mid 1960s (Figure 4) and the reduction in bladder cancer in chemical industry workers that occurred after a decrease in the use of 2-naphthylamine in that industry.
Based on this kind of evidence, epidemiologists have concluded that 75 to 80% of cancers in the United States are avoidable (Figure 5). A more detailed analysis of the available data provides a list of some of the avoidable causes of cancer:
- Use of tobacco cause of ~30% of preventable cancer deaths. This is the biggest preventable cause of cancer, responsible for ~90% and ~80% of lung cancer deaths in males and females, respectively, in the developed world. In addition, nonsmokers living with a smoker exhibit an up to 24% increased risk of lung cancer. Tobacco use contributes to the risk of many other forms of cancer and multiplies the risks of other cancer-causing agents, such as alcohol, asbestos, and ionizing radiation.
- Use of alcohol, cause of ~3% of preventable cancer deaths. Although not carcinogenic by itself, alcohol works together with smoking and other factors to markedly increase the rate of cancers of the pharynx, larynx, esophagus, and liver.
- Diet, cause of ~30% of preventable cancer deaths. Diet can influence the development of cancer in multiple ways, such as:
- The presence in some foods of small amounts of naturally occurring carcinogens or substances that can be converted to carcinogens in the body.
- The production of carcinogens in foods during cooking. This is particularly true for well-done cooked meats, smoked meats, and foods fried in oil that has been reused many times.
- The formation of carcinogens during storage under poor conditions. This is particularly important in developing countries where moldy grains and peanuts become contaminated with aflatoxin, a highly potent liver carcinogen.
- The use of some food additives. The recent decrease in cancer of the stomach is likely the result of a reduction in the use of high concentrations of salt to preserve food, which has been possible due to the availability of better food storage methods.
- The presence of dietary fiber, which reduces exposure to carcinogens produced by bacteria in the large intestine.
- Overnutrition. The relationship between obesity and multiple forms of cancer, including cancer of the endometrium, kidney, gall bladder, esophagus, colon, breast, and prostate, is just now coming to light, and we are only beginning to understand how obesity contributes to carcinogenesis.
- Infection, cause of ~5% of preventable cancer deaths. Infectious agents that directly or indirectly cause cancer include human papilloma virus (cervical cancer), hepatitis B and C viruses (liver cancer), Epstein Barr virus (Burkitt’s lymphoma), Helicobacter pylori bacteria (gastric carcinoma and lymphoma), and schistosome parasites (bladder cancer).
- Reproductive factors, cause of ~3% of preventable cancer deaths. Pregnancy and childbearing reduce the risk of endometrial, ovarian, and breast cancers.
- Occupational exposures, cause of <5% of preventable cancer deaths. These include some aromatic amines, asbestos, arsenic, vinyl chloride, and ionizing radiation. In most cases, the affected population is small, but the risk for that population is quite high.
- Environmental pollution, cause of ~2% of preventable cancer deaths. Most data that suggest a role for environmental pollutants implicate air pollution from the combustion of fossil fuels.
- Ionizing radiation, cause of ~2% of preventable cancer deaths. This includes ultraviolet radiation from the sun, the primary cause of skin cancer, and other sources of radiation, such as radon. The actual number of cancers caused by ultraviolet radiation is much higher than indicated by the percent of preventable deaths because the majority of skin cancers are detected early and effectively treated.
- Medical treatments, cause of ~1% of preventable cancer deaths. Ironically, many cancer chemotherapeutic drugs can, themselves, cause cancer. Exposure to X-rays and the use of estrogens for hormonal replacement therapy after menopause and in oral contraceptives are other medical interventions that carry some risk of cancer. In all of these cases, the benefit of therapy usually outweighs the cancer risk.
The identification of preventable causes of cancer can lead to personal choices and public policies that will reduce the incidence of cancer. However, this goal may not be as easy as it sounds. Clearly for some people, quitting smoking is extremely difficult, and the growing obesity epidemic underscores the challenges associated with promoting a healthy diet. Yet, some progress has been made, and as we understand more about how these various factors contribute to carcinogenesis, we may be able to find innovative ways to prevent their adverse effects on human health.
In 1971, President Richard Nixon declared war on cancer and sought $100 million to fund it. In 1972, Waddell Walker Hancock established the A. B. Hancock Jr. Memorial Laboratory for Cancer Research at Vanderbilt University Medical Center in honor of her husband, Bull. The memorial she created was the Medical Center’s first named laboratory dedicated to cancer research, and it laid the foundation for the establishment of the Vanderbilt-Ingram Cancer Center (VICC) in 1993. An alumna of Vanderbilt, Mrs. Hancock was a determined visionary who was dedicated to the university and the fight against cancer. She served on the Board of Overseers for the VICC, inspiring many to support the transformational research she was confident would lead to lifesaving discoveries.
In 2001, the Vanderbilt University Board of Trust approved $100 million in funding for the Academic Venture Capital Fund to recruit world-class faculty, invest in cutting-edge resources, strengthen educational programs at all levels, and establish dynamic multidisciplinary research programs. The Hancock Laboratory was a model for what the Board aimed to achieve. As the lab’s director since 1989, Larry Marnett, Ph.D., University Professor and Mary Geddes Stahlman Professor of Cancer Research, has led the way in leveraging philanthropic resources to forge innovative teams of stellar talent that are expanding the frontier of cancer research across institutions over the entire campus.
As the A. B. Hancock Jr. Memorial Laboratory for Cancer Research passes its 40th anniversary, we celebrate its seminal contributions to fundamental scientific breakthroughs in combatting cancer as well as its role in the development of passionate coalitions of researchers, a peerless Medical Center infrastructure of programs and equipment for biomedical research, and a strong pipeline for engaging and mentoring young scientists and physicians. Recent advances in scientific knowledge are propelling cancer research forward at an unprecedented rate. With the steadfast support of the Hancock family and friends, the Hancock Lab is prepared to make the most of these transformational opportunities. Its vision is to accelerate discovery by marshalling the resources of the lab in strategic ways and continuing to invest in innovative talent.
Modern medicine saves and extends lives. Yet all too often, a treatment that works in one patient does not in another. Or a drug that helps one person harms another. Researchers are making discoveries in genomics and chemical biology that are beginning to allow doctors to “tailor” treatments to individual patients — to give them what they need and what will work, while minimizing side effects. This is the science of personalized medicine, and it’s being pioneered at Vanderbilt.
Personalized cancer medicine is possible because scientists are increasingly able to identify and precisely characterize genetic diversity among individuals and to associate these variations with health or cancer. The National Cancer Institute-supported Cancer Genome Atlas is providing this information for cancer patients on a grand scale. In September 2010, the Vanderbilt-Ingram Cancer Center launched a Personalized Cancer Medicine Initiative, becoming one of the first cancer centers in the nation to offer routine genotyping of lung and melanoma tumors. Because of this, Vanderbilt physicians can now treat some tumors with a therapy specifically targeted to the genetic changes that are driving the cancer’s growth. By moving away from one-size-fits-all care, we are leading the fight against cancer to deliver the right care tailored to the right patient.
As we increasingly become aware of the complexity of cancer, it is also becoming evident that winning the battle against cancer requires a multidisciplinary approach. A key to the Hancock Lab’s success has been the strategy that Dr. Marnett developed twenty years ago of assembling multidisciplinary teams of clinicians and scientists to tackle critical problems in a focused manner. More relevant now than ever, this visionary strategy has fostered exciting collaborations within Vanderbilt and with top scientists around the world. For example:
- Because of chemical studies completed at Vanderbilt, new molecules have been evaluated at the University of Pennsylvania, and compounds are being tested at Harvard University for treatment of castrate-resistant prostate cancer. If successful, these compounds will provide a strategy to attack prostate cancer at its deadliest stage—after it has become resistant to first-line therapy.
- Another collaboration with investigators at Cornell University is designed to image inflammation in breast tissue of obese women. Since inflammation can lead to cancer, this technology enables physicians to identify lesions with the potential to become malignant. By taking cancer detection to an even earlier stage than is currently possible, we are attacking what is, perhaps, the biggest emerging challenge to our health care system - the rapidly increasing rate of obesity and its link to cancer.
- Vanderbilt is working with other members of the Translational Breast Cancer Research Consortium to test a new, targeted therapy against one form of triple negative breast cancer (TNBC), an approach suggested by our study of TNBC subtypes.
Located in the midst of one of the great medical centers and great cancer centers in the world, the Hancock Lab is a central player in energetic partnerships that are determined to improve cancer detection, prevention, and treatment. The future of the Hancock Laboratory will be intimately linked to dynamic collaboration across the disciplines of chemistry, molecular biology, and medicine with a singular purpose –– to provide the best ideas, technologies, and strategies for cancer detection, prevention, and treatment to each and every person who has cancer or is at-risk of developing cancer.
While we celebrate our advances, we are mindful of the work that lies ahead in reducing the cancer burden. Thus, in recognition of the 40th anniversary of the Hancock lab, we are working to establish a fund honoring Waddell Walker Hancock and her pursuit of innovation in cancer science. This fund would complement the Hancock lab and signify the momentum of the exciting new era of personalized cancer medicine.
An endowment for the Hancock Lab will allow us to form teams of investigators at Vanderbilt and collaborating institutions who will apply their complementary skills to important cancer problems as quickly and efficiently as possible. Rather than waiting one to two years for grants to be funded by government agencies, Hancock-supported investigators will be instantly testing their ideas in the lab and the clinic. These teams will develop and use the best science and technology to provide personalized approaches to cancer detection, prevention, and therapy, validating legitimate targets and optimizing new drug candidates through preclinical testing.
Our own institutional strengths combined with our ability to collaborate with partners around the world means that we will identify the fastest, most effective methods for advancing cancer science. As stated by Dr. Marnett, “We will go where the science leads us, within and beyond our walls to aggressively attack the most important problems in cancer.”
Staying true to the original intent of the laboratory as outlined by Waddell Walker Hancock—to produce life-saving discoveries—the Hancock Lab is, and will remain, a catalyst for drug discovery and improved cancer care.
Vanderbilt University is committed to principles of equal opportunity and affirmative action. | Copyright © 2013 by Vanderbilt University Medical Center