Structural insights into base excision repair
As the instruction manual of the cell, DNA plays an essential role in development and organism function. Consequently, several mechanisms exist to repair DNA following damage. Oxidants result in structural damage to DNA which are typically repaired via base excision repair (BER). A set of proteins called glycosylases serve to recognize these DNA lesions and initiate BER. hNEIL1, a human glycosylase, has been linked to a wide variety of DNA lesions but primarily studied in the context of thymine glycol (Tg) lesions. Furthermore, hNEIL1 has two isoforms, edited and 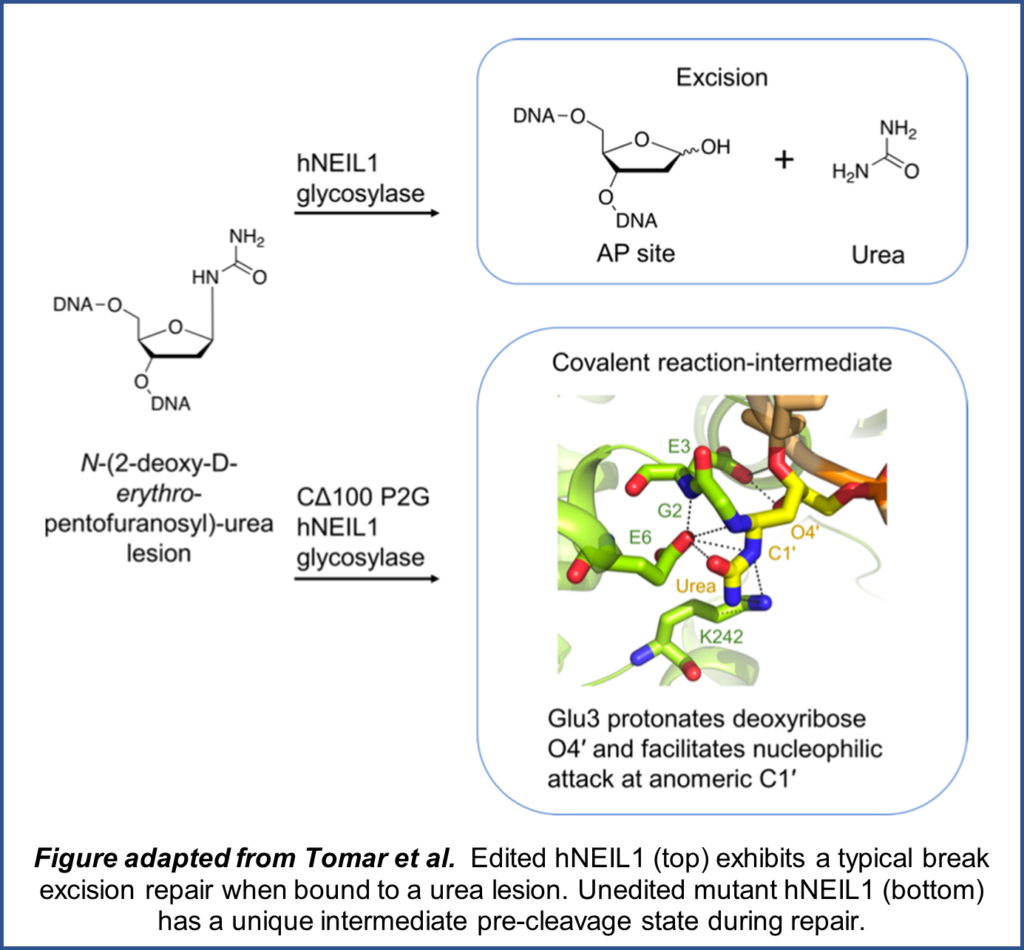 unedited, which act differentially on Tg lesions.
unedited, which act differentially on Tg lesions.
Rachana Tomar from the Stone lab, alongside collaborators in the Iverson and Egli labs, recently published a paper in Nucleic Acids Research that investigated both forms of hNEIL1 within the context of urea lesions. Using a combination of biochemical and structural techniques, they were able to determine the structures and activity of both glycosylase isoforms when bound to urea DNA lesions. While the unedited form of hNEIL1 exhibited a novel intermediate pre-cleavage state, there was no significant difference in efficiency of urea lesion removal between the two isoforms.
This study sheds light on the mechanism of hNEIL1 function between different DNA lesions and provides a basis for further research in base excision repair pathways.
Read the full article here! ~Cameron I. Cohen
Leave a Response
You must be logged in to post a comment