ASSIGNMENT |
A bottle of unlabeled inorganic salt has been found in the laboratory. Since times are tough, we have decided to determine its identity rather throw it out. We know it must be NaCl, KCl, NaNO3, NaBr, KBr, NaI, KI, since these are the only salts we use in our laboratory. Use ion-exchange chromatography to determine the identity of the salt.
CONCEPTS |
The task of the chemist often involves the analysis an unknown substance. For example, a chemist may be given a water sample from a stream and be asked to determine the types and amount of pollutants that are present. Another situation might involve a chemist determining the blood chemistry of an elderly patient suffering from Alzheimers Disease. These are just a few examples where a chemist is required to analyze and study an unknown and draw conclusions based on the results that he or she obtains.
There are many methods that a chemist can use to determine the identity of an unknown substance. Often these various method are used in combination with one another. For example, spectroscopy, detailed study of physical properties, and chemical analysis (cation and anion analysis and titration) can all be useful tools in identifying unknown substances. In most cases these methods can be used to directly study a substance. However, in other cases, it can be useful or even necessary to convert a compound into a different species in order to use these methods more efficiently.
One class of compounds which can be altered in order to simplify their analysis are inorganic salts. Inorganic salts can be quantitatively studied by titration through their conversion into acids or bases using ion-exchange resin. A commonly encountered example of the conversion of a salt using an ion-exchange resin is found in residential water softeners, Figure 1. In this process, water is softened by replacing the hard-water cations (Mg2+, Ca2+, Fe2+) with the water softened sodium ions. In a similar fashion, ion-exchange chromatography can be used to convert a known quantity of an unknown salt into an acid or a base.

There are two general principles involved in ion-exchange chromatography. These include the mobile phase and the stationary phase. In cation-exchange chromatography, the stationary phase, which consists of a large quantity of acid groups attached to a polymeric resin, is slurried with water and applied to a column. The mobile phase, which contains the inorganic salt dissolved in a suitable solvent, is applied to the column. As the mobile phase passes through the column, exchange between the H+ ions on the polymeric ion-exchange resin of the stationary phase and the cations of the salt in the mobile phase occur. The solution which is collected at the bottom of the column contains the acid form of the inorganic salt. The newly formed acidic solution can then titrated using a standardized base to determine the number of moles present in the sample. The molecular weight of the substance and the identity of the unknown can than be determined based on the number of moles and the weight of the sample.
CALCULATIONS |
As you will soon see in the technique section of this laboratory, this experiment requires that you weigh a certain mass of the unknown salt, dissolve it into a solvent, and pass it over a column of ion-exchange resin. As mentioned previously, this process serves the purpose of converting the salt into its corresponding acid. For example, KCl, LiCl, and NaCl would all be transformed to HCl. Once the salt has been converted, the acid solution which is a result of this procedure is titrated using a standardized solution of sodium hydroxide. With the information obtained from your titration, you can calculate the number of moles of NaOH used.
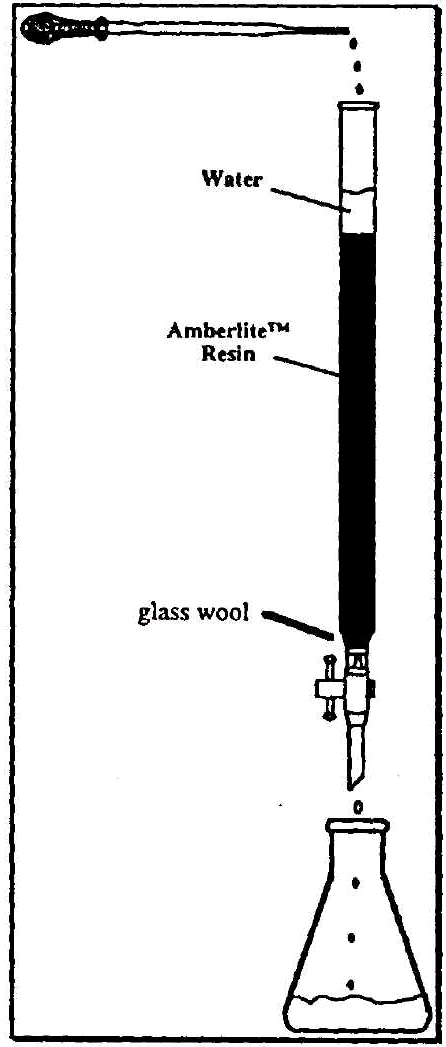
PROCEDURE A (Refer to Figure 2) |
PROCEDURE B |