Research Interests
Nanocrystals have been central to recent advances in green technologies, such as lithium ion batteries, fuel cells, and solar panels. In some cases, technologies that were not efficient or fast enough to be cost-effective have been granted new life through nano-sizing key components. Tiny nanocrystals do almost everything faster than their larger counterparts because tiny pieces present a larger amount of exposed surface. Therefore, any chemical process that happens on a surface is much faster because there is so much more surface.
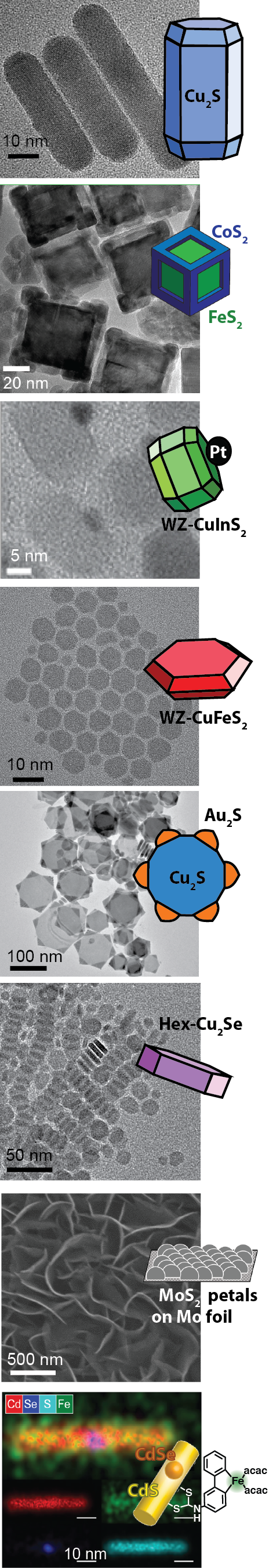
Lithium ions in batteries are absorbed and released more speedily when the cobalt oxide electrodes are broken into nano-sized pieces. For this reason, computer batteries now charge faster and weigh less because all of the host material can be accessed by the lithium ions racing in and out of the particle surfaces. In another example, the nano-sizing of the lead sulfide crystals that absorb light in a new design of solar cells has been central to its success. Electrons can hop readily out of the particles through the many surfaces to travel along electrical circuits. Furthermore, because nanocrystals can be suspended in liquids like inks, there is potential for lead sulfide solar panels to be manufactured through inexpensive spray processing. In our own research, molybdenum sulfide in large crystals is an ineffective electrode material with nearly zero activity for some solar cell applications, yet when the crystals are nano-sized flower petals (left figure, second to bottom), it becomes sixteen times more active than platinum (the gold standard of electrode materials).
The future is bright for further advances facilitated by nanocrystals. Being pursued are nanocrystals that can absorb light and turn it into electrical energy, facilitate precise chemical reactions on their surfaces in industrial settings, absorb or remediate toxins in air or water, or facilitate fuel cell reactions or carbon dioxide capture. Underpinning all this potential is the need to synthesize nanocrystals of new materials, designed for each of these applications. There are thousands of known types of inorganic crystals with constituent atoms from across the periodic table, but only a relative few have been synthesized as nanocrystals. Such a diversity in composition means there is equal diversity in the properties and function of these materials as nanocrystals. The untapped potential is enormous.
Nanocrystals are most often synthesized in a flask, suspended in solution like ink. In addition to the crystalline inorganic cores, each crystal has a coating of organic molecules to prevent the crystals from growing beyond nano-size. The properties of nanocrystals are very dependent on the atomic make-up of the crystalline core and the surface coating. It is only by fully understanding and synthetically controlling both components that further technological advances with nanocrystals can be made.
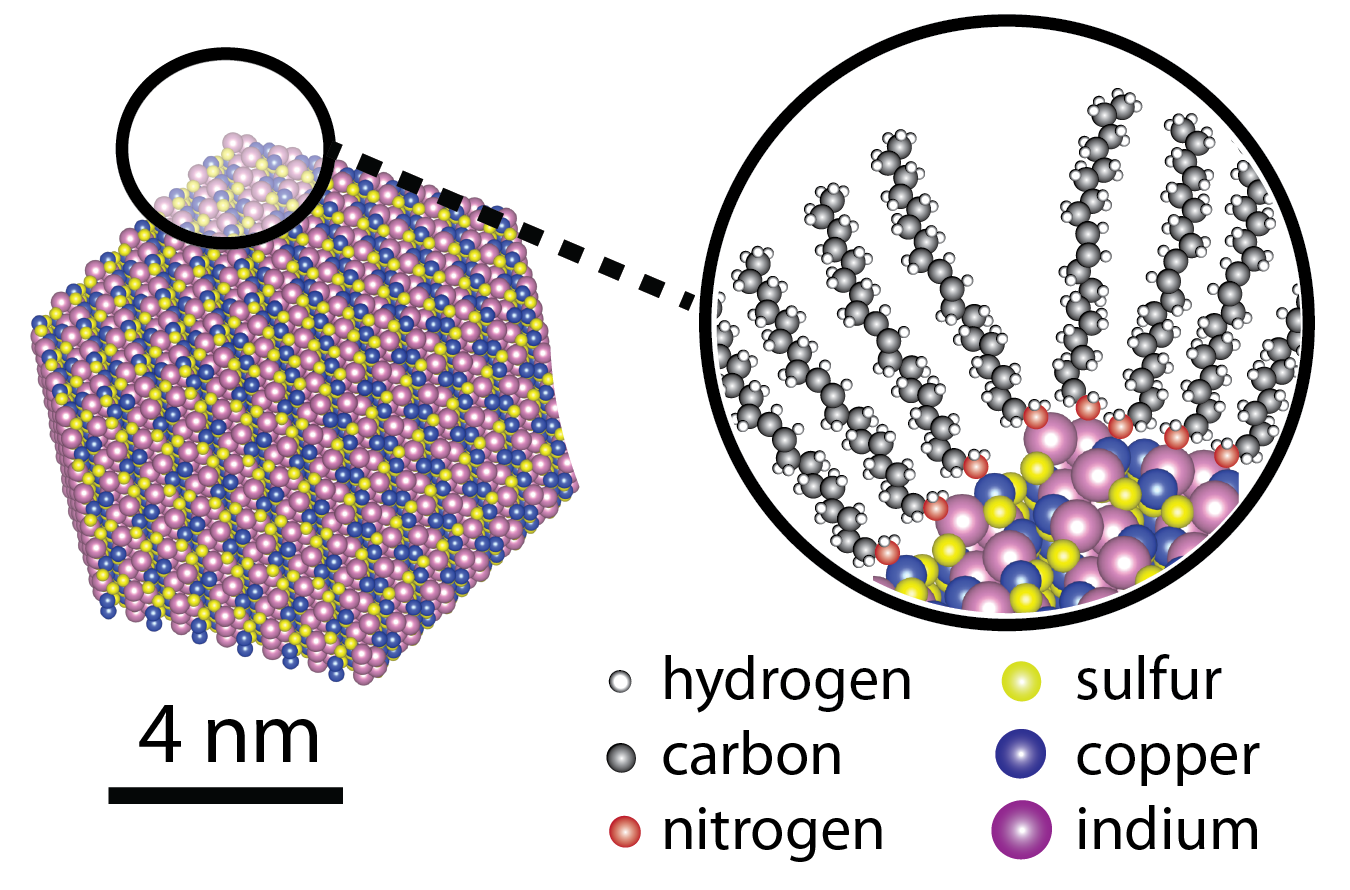
My research program is in the synthesis of nanocrystals. Inspired by the astounding diversity of crystals in the geologic record, we work to understand the fundamental synthetic principles needed to prepare such crystals in the laboratory on the nanoscale and apply these principles to prepare unnatural crystal types that were previously unknown. We strive to understand and exploit the complex interactions of the organic ligand coating with the surfaces of the nanocrystals and how that influences the synthesis, physical properties, and eventual function of the nanocrystals.
I believe in a collaborative approach to advance our ambitious goals, which is why my team has been working extensively with theoretical physicists at Vanderbilt and Lawrence Berkeley National Laboratory to model the nanocrystal surfaces and their electronic structure and defects; electron microscopists at Vanderbilt and at Oak Ridge National Laboratory to obtain atomic resolution images of the nanocrystals; and spectroscopists at Vanderbilt and at The Hebrew University of Jerusalem in Israel to measure the intimate details about how the new nanocrystals we are synthesizing interact with light. We leverage Vanderbilt’s resources by heavily utilizing the characterization tools at the Vanderbilt Institute for Nanoscale Science and Engineering (VINSE) such as the electron microscopes, and more recently the small molecule NMR core for in situ NMR experiments with non-standard nuclei.

